Azacitidine (5-Azacytidine)
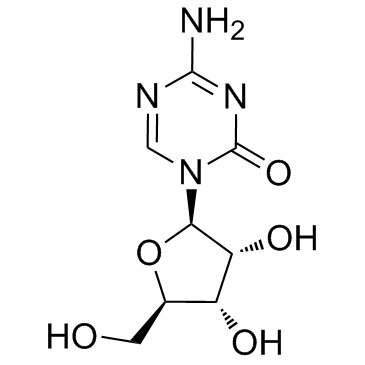
Azacitidine (5-Azacytidine) structure
|
Common Name | Azacitidine (5-Azacytidine) | ||
|---|---|---|---|---|
| CAS Number | 320-67-2 | Molecular Weight | 244.205 | |
| Density | 2.1±0.1 g/cm3 | Boiling Point | 534.5±60.0 °C at 760 mmHg | |
| Molecular Formula | C8H12N4O5 | Melting Point | 226-232 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 277.0±32.9 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of Azacitidine (5-Azacytidine)5-Azacytidine is a nucleoside analogue of cytidine that specifically inhibits DNA methylation by trapping DNA methyltransferases. |
| Name | 5-azacytidine |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Azacytidine is a nucleoside analogue of cytidine that specifically inhibits DNA methylation by trapping DNA methyltransferases. |
|---|---|
| Related Catalog | |
| Target |
DNMT1 Nucleoside Antimetabolite/Analog Autophagy |
| In Vitro | Unmethylated CpG islands associated with a variety of genes become partially or fully methylated in tumors and can be reactivated by 5-Azacytidine[1]. 5-Azacytidine acts as weak inducers of erythroid differentiation of Friend erythroleukemia cells in the same concentration range where they affect DNA methyltransferase activity[2]. 5-Azacytidine inhibits L1210 cells with ID50 and ID90 values of 0.019 and circa 0.15 μg/mL, respectively[3]. |
| In Vivo | TdR-3H incorporation is significantly inhibited when the animals are exposed to 5-Azacitidine (100 mg/kg, i.p.) for 2 hr or longer[3]. |
| Kinase Assay | A crude cell-free extract is isolated from LI 210 cells in culture by suspension of the cells in a given volume of 0.05mol/LTris-HCl buffer, pH 7.4, and sonic extraction with a Biosonik at 70% maximal output for 30 sec. The supernatant is collected after centrifugation at 105,000 × g for 60 min (4°C) in a Model L Spinco ultracentrifuge. The final protein concentration of the cell-free extracts is approximately 3 mg/mL. The extracts are used as the source of enzymes. Ribonucleotide reductase activity is measured. A unit of enzyme is defined as the amount that catalyzed dCMP synthesis at a rate of 1 mμmole/hr. The assay systems for the measurement of pyrimidine nucleoside (CR) and deoxynucleoside (TdR, CdR) kinases are essentially those described by Chu and Fischer. However, reactions are terminated by heating for 2 min in a boiling water bath, and the phosphorylated derivatives are isolated according to the method of Bach. Fifty-jul aliquots are applied to 1-inch discs of diethylaminoethyl paper, which are then placed in counting vials and eluted with 0.5 mL of 0.5 mol/LPCA. After 1 hr, 12 mL of Diotol are added, and the radioactivity is determined. |
| Cell Assay | Twenty mL of cells (circa 1×104 cells/mL) are pipetted into sterilized culture tubes with screw caps and incubated at 37°C overnight. The experiment is initiated by the addition of 1 mL of 5-Azacytidine (5-azaCR) or medium for a given period (from 0 to 240 min) prior to the addition of 1 mL of metabolite (or medium). Cell growth is determined twice a day for 3 days by means of a Model A Coulter counter. To determine IDSO and ID90 values, 5 mL of L1210 cells (5×103 cells/mL) are incubated with the drug at 37°C for 3 days, and cell growth is determined. |
| Animal Admin | For the in vivo experiments, leukemic mice (bearing circa 1×103 cells/animal) are given injections i.p. with 0.2 mL of 5-Azacytidine (5-azaCR) of a given concentration. Two hr later, the reaction is started by injecting 0.5 mL of labeled metabolite (TdR-3H or UR-3H, 10 /μCi/12.5 μg). After 1 hr, animals (3 mice/group) are killed by cervical fracture, and the ascites are treated with heparin, collected, pooled, and then centrifuged immediately in a Sorvall refrigerated centrifuge Model R2C-B at 800×g for 10 min (4°C). |
| References |
| Density | 2.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 534.5±60.0 °C at 760 mmHg |
| Melting Point | 226-232 °C (dec.)(lit.) |
| Molecular Formula | C8H12N4O5 |
| Molecular Weight | 244.205 |
| Flash Point | 277.0±32.9 °C |
| Exact Mass | 244.080765 |
| PSA | 143.72000 |
| LogP | -1.99 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.823 |
| InChIKey | NMUSYJAQQFHJEW-KVTDHHQDSA-N |
| SMILES | Nc1ncn(C2OC(CO)C(O)C2O)c(=O)n1 |
| Water Solubility | 0.5-1.0 g/100 mL at 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H350 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R45;R46;R22 |
| Safety Phrases | S53-S22-S36/37/39-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | XZ3017500 |
| HS Code | 2934999090 |
|
~71% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: ScinoPharm Taiwan Ltd. Patent: US2010/36112 A1, 2010 ; Location in patent: Page/Page column 7 ; |
|
~40% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: Ionescu, Dumitru; Blumbergs, Peter Patent: US2004/186283 A1, 2004 ; Location in patent: Page 8 ; |
|
~75% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: SICOR INC.; BIGATTI, Ettore; LUX, Giovanna; PAIOCCHI, Maurizio; GIOLITO, Andrea; TOSI, Simone Patent: WO2010/17374 A1, 2010 ; Location in patent: Page/Page column 18 ; |
|
~% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: EP2371825 A1, ; Page/Page column 9 ; |
|
~% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: EP2371825 A1, ; Page/Page column 9 ; |
|
~% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: US2014/135490 A1, ; Page/Page column ; |
|
~75% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: Vujjini, Satish Kumar; Varanasi, Ganesh; Arevelli, Srinivas; Kandala, Sreenatha Charyulu; Tirumalaraju, Satyanarayana Raju; Bandichhor, Rakeshwar; Kagga, Mukkanti; Cherukupally, Praveen Organic Process Research and Development, 2013 , vol. 17, # 2 p. 303 - 306 |
|
~% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: WO2009/16617 A2, ; Page/Page column 15-16 ; |
|
~% 
Azacitidine (5-... CAS#:320-67-2 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 70, # 11 p. 1228 - 1232 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The high mobility group A2 protein epigenetically silences the Cdh1 gene during epithelial-to-mesenchymal transition.
Nucleic Acids Res. 43(1) , 162-78, (2015) The loss of the tumour suppressor E-cadherin (Cdh1) is a key event during tumourigenesis and epithelial-mesenchymal transition (EMT). Transforming growth factor-β (TGFβ) triggers EMT by inducing the e... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
| Vidaza |
| 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxyméthyl)tétrahydrofuran-2-yl]-1,3,5-triazin-2(1H)-one |
| EINECS 206-280-2 |
| [14C]-Azacitidine |
| 5 AZC |
| 4-Amino-1-b-D-ribofuranosyl-1,3,5-triazine-2(1H)-one |
| 5-AZCR |
| 1,3,5-Triazin-2(1H)-one, 4-amino-1-β-D-ribofuranosyl- |
| 5'-azacytidine |
| azacytidine |
| Ladakamycin |
| Azacitidine |
| AzGR |
| 5-Azacytidine |
| 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,3,5-triazin-2(1H)-one |
| 5-AC |
| 4-amino-1-(b-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one |
| 5-AC-15N4 |
| 4-Amino-1-(β-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one |
| 4-Amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-1,3,5-triazin-2(1H)-one |
| L-β-Ribofuranosyl-5-azacytosine |
| 4-Amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,3,5-triazin-2(1H)-on |
| 1,3,5-triazin-2(1H)-one, 4-amino-1-b-D-ribofuranosyl- |
| mylosar |
| Azacytidine, 5- |
| MFCD00006539 |
| 5-AZAC |
![N-(Trimethylsilyl)-4-[(trimethylsilyl)oxy]-1,3,5-triazin-2-amine structure](https://image.chemsrc.com/caspic/089/52523-35-0.png)

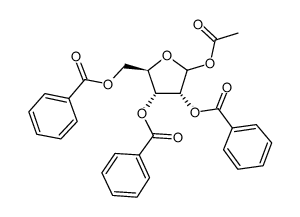

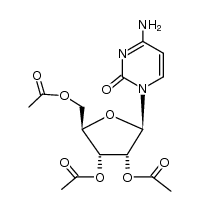
![N-[amino-[[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]carbamoylamino]methylidene]formamide structure](https://image.chemsrc.com/caspic/041/65126-88-7.png)
![4-amino-1-[4-(hydroxymethyl)-7,7-dimethyl-3,6,8-trioxabicyclo[3.3.0]oct-2-yl]-1,3,5-triazin-2-one structure](https://image.chemsrc.com/caspic/217/65370-90-3.png) CAS#:65370-90-3
CAS#:65370-90-3
