Atorvastatin hemicalcium trihydrate
Modify Date: 2025-08-25 10:10:11
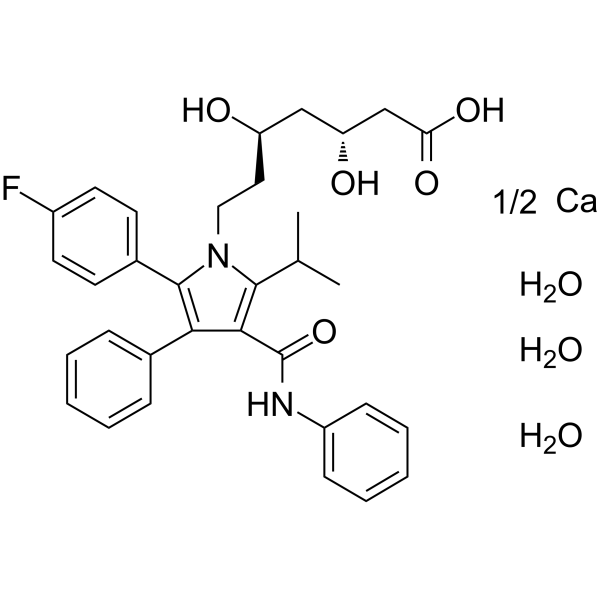
Atorvastatin hemicalcium trihydrate structure
|
Common Name | Atorvastatin hemicalcium trihydrate | ||
|---|---|---|---|---|
| CAS Number | 344920-08-7 | Molecular Weight | 632.73 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C33H35FN2O5.1/2Ca.3H2O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Atorvastatin hemicalcium trihydrateAtorvastatin hemicalcium trihydrate is an orally active HMG-CoA reductase inhibitor, has the ability to effectively decrease blood lipids. Atorvastatin hemicalcium trihydrate inhibits human SV-SMC proliferation and invasion with IC50s of 0.39 μM and 2.39 μM, respectively[1][2][3]. |
| Name | Atorvastatin hemicalcium trihydrate |
|---|
| Description | Atorvastatin hemicalcium trihydrate is an orally active HMG-CoA reductase inhibitor, has the ability to effectively decrease blood lipids. Atorvastatin hemicalcium trihydrate inhibits human SV-SMC proliferation and invasion with IC50s of 0.39 μM and 2.39 μM, respectively[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Atorvastatin hemicalcium trihydrate treatment decreases apoptosis of myocardial cells by down-regulating GRP78, caspase-12 and CHOP expression in myocardial cells after myocardial infarction, and the endoplasmic reticulum (ER) stress is activated in response to heart failure and angiotensin II (Ang II) stimulation[4]. |
| In Vivo | Atorvastatin (20-30 mg/kg; oral gavage; once a day; for 28 days; ApoE−/− mice) hemicalcium trihydrate treatment significantly reduces endoplasmic reticulum (ER) stress signaling proteins, the number of apoptotic cells, and the activation of Caspase12 and Bax in the Ang II-induced ApoE-/- mice. Proinflammatory cytokines such as IL-6, IL-8, IL-1β are all remarkably inhibited after Atorvastatin treatment[5]. Animal Model: Forty 8-week-old ApoE−/− mice induced with angiotensin II (Ang II)[5] Dosage: 20 mg/kg, 30 mg/kg Administration: Oral gavage; once a day; for 28 days Result: Significantly reduced ER stress signaling proteins, the number of apoptotic cells, and the activation of Caspase12 and Bax in the Ang II-induced ApoE−/− mice. Proinflammatory cytokines such as IL-6, IL-8, IL-1β were all remarkably inhibited. |
| References |
| Molecular Formula | C33H35FN2O5.1/2Ca.3H2O |
|---|---|
| Molecular Weight | 632.73 |
| InChIKey | KQPCBHBPIZWHAS-NGUMWDRNSA-L |
| SMILES | CC(C)c1c(C(=O)Nc2ccccc2)c(-c2ccccc2)c(-c2ccc(F)cc2)n1CCC(O)CC(O)CC(=O)[O-].CC(C)c1c(C(=O)Nc2ccccc2)c(-c2ccccc2)c(-c2ccc(F)cc2)n1CCC(O)CC(O)CC(=O)[O-].O.O.O.O.O.O.[Ca+2] |