3′-(Trifluoromethyl)acetophenone
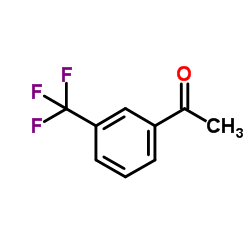
3′-(Trifluoromethyl)acetophenone structure
|
Common Name | 3′-(Trifluoromethyl)acetophenone | ||
|---|---|---|---|---|
| CAS Number | 349-76-8 | Molecular Weight | 188.146 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 199.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C9H7F3O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 83.9±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 1-[3-(trifluoromethyl)phenyl]ethanone |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 199.0±0.0 °C at 760 mmHg |
| Molecular Formula | C9H7F3O |
| Molecular Weight | 188.146 |
| Flash Point | 83.9±0.0 °C |
| Exact Mass | 188.044907 |
| PSA | 17.07000 |
| LogP | 2.72 |
| Vapour Pressure | 0.3±0.3 mmHg at 25°C |
| Index of Refraction | 1.447 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| HS Code | 29147090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
Highly enantioselective catalytic phenylation of ketones with a constrained geometry titanium catalyst.
Org. Lett. 5(20) , 3641-4, (2003) [reaction: see text] The catalytic asymmetric addition of phenyl groups from diphenylzinc to ketones is reported. The catalyst, generated from a dihydroxy bis(sulfonamide) ligand and titanium tetraiso... |
|
|
Asymmetric transfer hydrogenation: chiral ligands and applications.
Chem. Soc. Rev. 35(3) , 226-36, (2006) Hydrogen transfer reduction processes are attracting increasing interest from synthetic chemists in view of their operational simplicity and high selectivity. In this tutorial review the most signific... |
|
|
A practical catalytic asymmetric addition of alkyl groups to ketones.
J. Am. Chem. Soc. 124(37) , 10970-1, (2002) Many catalysts will promote the asymmetric addition of alkylzinc reagents to aldehydes. In contrast, there are no reports of additions to ketones that are both general and highly enantioselective. We ... |
| 1-Acetyl-3-(trifluoromethyl)benzene |
| 3’-(Trifluoromethyl)acetophenone |
| Acetophenone, 3'-(trifluoromethyl)- |
| m-Trifluoromethylacetophenone |
| 1-[3-(Trifluoromethyl)phenyl]ethanone |
| 3′-(Trifluoromethyl)acetophenone |
| FXFFR CV1 |
| Ethanone, 1-[3-(trifluoromethyl)phenyl]- |
| EINECS 206-490-4 |
| 3'-(TrifluoroMethyl)acetophenone |
| 3‘-(Trifluoromethyl)acetophenone |
| 1-[3-(trifluoromethyl)phenyl]ethan-1-one |
| MFCD00000391 |
| 1-[3-(Trifluoromethyl)phenyl]-1-ethanone |
| 3-Acetylbenzotrifluoride |
| 1-(3-trifluoromethyl-phenyl)-ethanone |
| 1-(3-(Trifluoromethyl)phenyl)ethanone |
| 3-(Trifluoromethyl)acetophenone |
![1-[3-(Trifluoromethyl)phenyl]ethanol Structure](https://image.chemsrc.com/caspic/458/454-91-1.png) CAS#:454-91-1
CAS#:454-91-1 CAS#:49623-20-3
CAS#:49623-20-3 CAS#:401-78-5
CAS#:401-78-5 CAS#:75-36-5
CAS#:75-36-5 CAS#:401-81-0
CAS#:401-81-0 CAS#:108-24-7
CAS#:108-24-7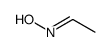 CAS#:5780-37-0
CAS#:5780-37-0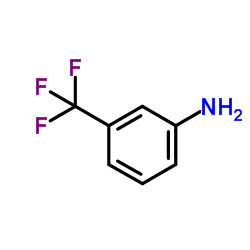 CAS#:98-16-8
CAS#:98-16-8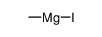 CAS#:917-64-6
CAS#:917-64-6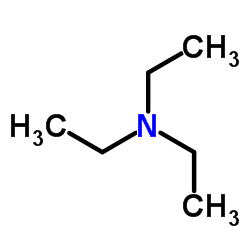 CAS#:121-44-8
CAS#:121-44-8 CAS#:67-64-1
CAS#:67-64-1 CAS#:454-92-2
CAS#:454-92-2![1-[3-(Trifluoromethyl)phenyl]ethanamine structure](https://image.chemsrc.com/caspic/466/59382-36-4.png) CAS#:59382-36-4
CAS#:59382-36-4 CAS#:38923-38-5
CAS#:38923-38-5 CAS#:351-35-9
CAS#:351-35-9 CAS#:99705-50-7
CAS#:99705-50-7 CAS#:59770-96-6
CAS#:59770-96-6 CAS#:705-28-2
CAS#:705-28-2
