INCA-6
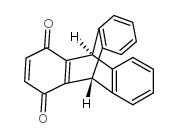
INCA-6 structure
|
Common Name | INCA-6 | ||
|---|---|---|---|---|
| CAS Number | 3519-82-2 | Molecular Weight | 284.30800 | |
| Density | 1.38g/cm3 | Boiling Point | 464.2ºC at 760 mmHg | |
| Molecular Formula | C20H12O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 172.6ºC | |
Use of INCA-6INCA-6 (Triptycene-1,4-quinone) is a cell-permeable NFAT inhibitor. INCA-6 specifically blocks targeting of NFAT(P) substrate to the calcineurin (CN) phosphatase site and is an effective inhibitor of CN-NFAT signaling[1][2][3]. |
| Name | inca-6 |
|---|---|
| Synonym | More Synonyms |
| Description | INCA-6 (Triptycene-1,4-quinone) is a cell-permeable NFAT inhibitor. INCA-6 specifically blocks targeting of NFAT(P) substrate to the calcineurin (CN) phosphatase site and is an effective inhibitor of CN-NFAT signaling[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | INCA-6 (5 μM; for 24-hour) prevents transient outward K+ current (Ito) downregulation in 3-Hz cells[1]. Pre-treatment of BV-2 cells with INCA-6 (10 μM) significantly inhibits ATP-induced CXCL2 expression in BV-2 cells. INCA-6 also inhibits ATP-induced CXCL2 expression in rat primary microglia[2]. INCA-6 (5 μM) reduces SERCA2 transcript levels as well as protein expression, in the absence or in the presence of thapsigargin (TG)[3]. INCA-6 (1.0 and 2.5 μM; 24 hours ) treatment significantly decreases both VEGF and serum-induced human retinal microvascular endothelial cells (HRMEC) proliferation, but does not affect baseline proliferation[4]. Cell Proliferation Assay[4] Cell Line: Human retinal microvascular endothelial cells Concentration: 0.5, 1.0, or 2.5 μM Incubation Time: 24 hours Result: Significantly inhibited VEGF-induced proliferation at 1.0 and 2.5 μM concentrations. |
| In Vivo | INCA-6 (5.0, or 25.0 μM) treatment significantly reduces pathologic neovascularization in oxygen-induced retinopathy (OIR)[4]. Animal Model: Rats bearing OIR model[4] Dosage: 2.5, 5.0, or 25.0 μM Administration: Intravitreal injection on days 14(0) and 14(3) Result: Decreased the severity of OIR in a dose dependent manner. Significant inhibition was seen at 5.0 and 25.0 μM concentrations. |
| References |
| Density | 1.38g/cm3 |
|---|---|
| Boiling Point | 464.2ºC at 760 mmHg |
| Molecular Formula | C20H12O2 |
| Molecular Weight | 284.30800 |
| Flash Point | 172.6ºC |
| Exact Mass | 284.08400 |
| PSA | 34.14000 |
| LogP | 3.28200 |
| Index of Refraction | 1.729 |
| InChIKey | GCHPUOHXXCNSQL-UHFFFAOYSA-N |
| SMILES | O=C1C=CC(=O)C2=C1C1c3ccccc3C2c2ccccc21 |
| Hazard Codes | Xi |
|---|
|
~84% 
INCA-6 CAS#:3519-82-2 |
| Literature: Zhu, Xiao-Zhang; Chen, Chuan-Feng Journal of Organic Chemistry, 2005 , vol. 70, # 3 p. 917 - 924 |
|
~% 
INCA-6 CAS#:3519-82-2 |
| Literature: Bartlett; Ryan; Cohen Journal of the American Chemical Society, 1942 , vol. 64, p. 2649,2651 |
|
~% 
INCA-6 CAS#:3519-82-2 |
| Literature: Bartlett; Ryan; Cohen Journal of the American Chemical Society, 1942 , vol. 64, p. 2649,2651 |
|
~% 
INCA-6 CAS#:3519-82-2 |
| Literature: Clar Chemische Berichte, 1931 , vol. 64, p. 1676,1679 |
|
~% 
INCA-6 CAS#:3519-82-2 |
| Literature: Clar Chemische Berichte, 1931 , vol. 64, p. 1676,1679 |
| NFAT Activation Inhibitor III |
| triptycene quinone |
| Inhibitor of NFAT-Calcineurin Association-6 |
| triptycene-1,4-quinone |
| triptycene-quinine |
| 9,10-dihydro-9,10-o-benzenoanthracene-1,4-dione |
![pentacyclo[6.6.6.0(2,7).0(9,14).0(15,20)]icosa-4,9,11,13,15,17,19-heptaene-3,6-dione structure](https://image.chemsrc.com/caspic/106/60835-52-1.png)
![9,10-dihydro-9,10-[o]-benzenoanthracene-1,4-diol structure](https://image.chemsrc.com/caspic/406/5969-70-0.png)
 CAS#:123-31-9
CAS#:123-31-9