SU3327
Modify Date: 2025-08-26 08:43:42
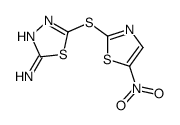
SU3327 structure
|
Common Name | SU3327 | ||
|---|---|---|---|---|
| CAS Number | 40045-50-9 | Molecular Weight | 261.30500 | |
| Density | 1.888g/cm3 | Boiling Point | 549.841ºC at 760 mmHg | |
| Molecular Formula | C5H3N5O2S3 | Melting Point | 160 °C(dec.) | |
| MSDS | N/A | Flash Point | 286.334ºC | |
Use of SU3327SU3327 is a potent, selective and substrate-competitive JNK inhibitor with an IC50 of 0.7 μM. SU3327 also inhibits protein-protein interactions between JNK and JNK Interacting Protein (JIP) with an IC50 of 239 nM. SU3327 shows less active against p38α and Akt kinase[1][2]. |
| Name | 5-[(5-nitro-1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazol-2-amine |
|---|---|
| Synonym | More Synonyms |
| Description | SU3327 is a potent, selective and substrate-competitive JNK inhibitor with an IC50 of 0.7 μM. SU3327 also inhibits protein-protein interactions between JNK and JNK Interacting Protein (JIP) with an IC50 of 239 nM. SU3327 shows less active against p38α and Akt kinase[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.7 μM (JNK); 239 nM (JNK-JIP interactions)[1] |
| In Vitro | SU3327 (compound 9) is able to inhibit TNF-α stimulated phosphorylation of c-Jun in HeLa cells (EC50 = 6.23 μM)[1]. SU3327 (25 nM) pretreatment of human-derived cerebral microvascular endothelial cells (hCMEC/D3) effectively reduces LPS-induced polymorphonuclear leukocytes (PMN) rolling/adhesion to hCMEC/D3, prevents activation of AP-1, and significantly reduces expression of VCAM-1[3]. |
| In Vivo | SU3327 (Compound 9; 25 mg/kg; intraperitoneal injection; male BKS.Cg-+Leprdb/+Leprdb/OlaHsd db/db mice) treatment possesses the ability to restore insulin sensitivity in mice models of diabetes[1]. SU3327 (Compound 9) has favorable microsomal and plasma stability (T1/2 = 27 min)[1]. Animal Model: Male BKS.Cg-+Leprdb/+Leprdb/OlaHsd db/db mice (11-week-old ) injected with insulin[1] Dosage: 25 mg/kg Administration: Intraperitoneal injection Result: Resulted in a statistically significant reduction in blood glucose levels. |
| References |
| Density | 1.888g/cm3 |
|---|---|
| Boiling Point | 549.841ºC at 760 mmHg |
| Melting Point | 160 °C(dec.) |
| Molecular Formula | C5H3N5O2S3 |
| Molecular Weight | 261.30500 |
| Flash Point | 286.334ºC |
| Exact Mass | 260.94500 |
| PSA | 193.02000 |
| LogP | 2.08950 |
| Index of Refraction | 1.793 |
| InChIKey | NQQBNZBOOHHVQP-UHFFFAOYSA-N |
| SMILES | Nc1nnc(Sc2ncc([N+](=O)[O-])s2)s1 |
|
~92% 
SU3327 CAS#:40045-50-9 |
| Literature: Bourdais; Dauvillier; Gayral; et al. European Journal of Medicinal Chemistry, 1981 , vol. 16, # 3 p. 233 - 239 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| Loreclezole hydrochloride |
| 2-amino-1,3,4-thiadiazole,9 |
| SU 3327 |
| 5-[(5-nitro-1,3-thiazol-2-yl)thio]-1,3,4-thiadiazol-2-amine |
| 5-(5-nitrothiazol-2-ylthio)-1,3,4-thiadiazol-2-amine |
| 5-(5-nitro-thiazol-2-ylsulfanyl)-[1,3,4]thiadiazol-2-ylamine |
| (amino-5 thiadiazol-1,3,4-yl-2) thio-2 nitro-5 thiazole |
| 2-Amino-5-(5-nitrothiazol-2-yl)mercapto-1,3,4-thiadiazole |