Qc 1
Modify Date: 2025-09-14 22:13:24
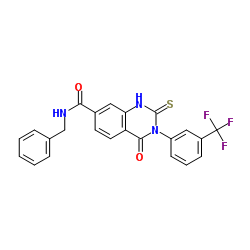
Qc 1 structure
|
Common Name | Qc 1 | ||
|---|---|---|---|---|
| CAS Number | 403718-45-6 | Molecular Weight | 455.452 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C23H16F3N3O2S | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of Qc 1Qc1 is a reversible and noncompetitive threonine dehydrogenase (TDH) inhibitor. Qc1 can be used for the research of Metabolic disease[1][2]. |
| Name | N-Benzyl-4-oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-1,2,3,4-tetrahydro-7-quinazolinecarboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | Qc1 is a reversible and noncompetitive threonine dehydrogenase (TDH) inhibitor. Qc1 can be used for the research of Metabolic disease[1][2]. |
|---|---|
| Related Catalog | |
| Target |
TDH[1] |
| In Vitro | Qc1 (10 μM; 2~24 hours; ES cells) shows that a substantial fraction of the total LC3 protein is in the LC3-I (cytoplasmic) form. After 16 h of TDH inhibition, most LC3 is converted to the LC3-II (lipid-modified) form, indicative of increased autophagic activity[2]. . Qc1 blocks the charging of tetrahydrofolate. Qc1 prevents threonine dehydrogenase from catabolizing threonine into acetyl-CoA and glycine[1][2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C23H16F3N3O2S |
| Molecular Weight | 455.452 |
| Exact Mass | 455.091522 |
| PSA | 98.98000 |
| LogP | 4.34 |
| Index of Refraction | 1.682 |
| InChIKey | IFNVTSPDMUUAFY-UHFFFAOYSA-N |
| SMILES | O=C(NCc1ccccc1)c1ccc2c(=O)n(-c3cccc(C(F)(F)F)c3)c(=S)[nH]c2c1 |
| RIDADR | NONH for all modes of transport |
|---|
| N-Benzyl-4-oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-1,2,3,4-tetrahydro-7-quinazolinecarboxamide |
| 7-Quinazolinecarboxamide, 1,2,3,4-tetrahydro-4-oxo-N-(phenylmethyl)-2-thioxo-3-[3-(trifluoromethyl)phenyl]- |