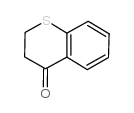
PD 146176
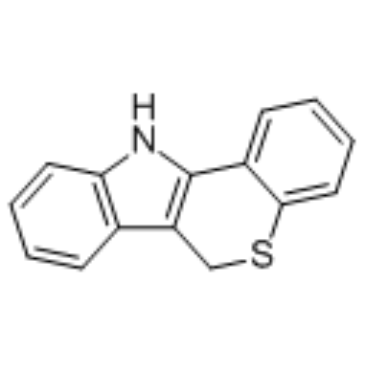
PD 146176 structure
|
Common Name | PD 146176 | ||
|---|---|---|---|---|
| CAS Number | 4079-26-9 | Molecular Weight | 237.32 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 485.4±24.0 °C at 760 mmHg | |
| Molecular Formula | C15H11NS | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 247.4±22.9 °C | |
Use of PD 146176PD146176 (NSC168807) is a 15-Lipoxygenase (15-LO) inhibitor, which inhibits rabbit reticulocyte 15-LO with a Ki of 197 nM. PD146176 (NSC168807) has a dramatic effect in reducing atherogenesis[1]. |
| Name | 6,11-dihydrothiochromeno[4,3-b]indole |
|---|---|
| Synonym | More Synonyms |
| Description | PD146176 (NSC168807) is a 15-Lipoxygenase (15-LO) inhibitor, which inhibits rabbit reticulocyte 15-LO with a Ki of 197 nM. PD146176 (NSC168807) has a dramatic effect in reducing atherogenesis[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 197 nM (Rabbit reticulocyte 15-LO)[1] |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 485.4±24.0 °C at 760 mmHg |
| Molecular Formula | C15H11NS |
| Molecular Weight | 237.32 |
| Flash Point | 247.4±22.9 °C |
| Exact Mass | 237.061218 |
| PSA | 41.09000 |
| LogP | 4.38 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.774 |
| InChIKey | ZGOOPZVQMLHPFM-UHFFFAOYSA-N |
| SMILES | c1ccc2c(c1)SCc1c-2[nH]c2ccccc12 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
|
~77% 
PD 146176 CAS#:4079-26-9 |
| Literature: Kidwai, M. M.; Ahluwalia, V. K. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1988 , vol. 27, # 1-12 p. 962 |
|
~% 
PD 146176 CAS#:4079-26-9 |
| Literature: Kucherova,N.F. et al. J. Gen. Chem. USSR (Engl. Transl.), 1963 , vol. 33, p. 3662 - 3667,3593 - 3597 |
|
~% 
PD 146176 CAS#:4079-26-9 |
| Literature: Kiang; Mann Journal of the Chemical Society, 1951 , p. 1909,1913 |
|
~% 
PD 146176 CAS#:4079-26-9 |
| Literature: Kiang; Mann Journal of the Chemical Society, 1951 , p. 1909,1913 |
|
~% 
PD 146176 CAS#:4079-26-9 |
| Literature: Kiang; Mann Journal of the Chemical Society, 1951 , p. 1909,1913 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity.
Neoplasia 12 , 254-63, (2010) The selenoenzyme glutathione peroxidase 4 (GPx4) has been described to control specific cyclooxygenases (COXs) and lipoxygenases (LOXs) that exert substantiated functions in tumor growth and angiogene... |
|
|
An enzyme that inactivates the inflammatory mediator leukotriene b4 restricts mycobacterial infection.
PLoS ONE 8 , e67828, (2013) While tuberculosis susceptibility has historically been ascribed to failed inflammation, it is now known that an excess of leukotriene A4 hydrolase (LTA4H), which catalyzes the final step in leukotrie... |
| indole<3,2-c>-1-thiochromen |
| 6,11-Dihydro<benzothiopyrano<6,3-b]indole |
| 6,11-Dihydro-<1>benzothiopyrano-<4,3-b>indol |
| 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole |
| 6,11-Dihydrothiochromeno[4,3-b]indole |
| 6,11-dihydro-5-thia-11-aza-benzo(a)-fluorene |
| [1]Benzothiopyrano[4,3-b]indole, 6,11-dihydro- |
| NAN-190 hydrobromide |
| 6,11-Dihydro-benzo<b>indolo<2,3-d>thiopyran |