H-1152
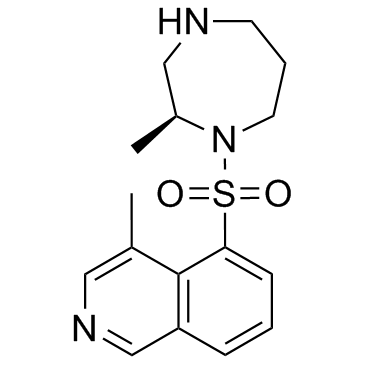
H-1152 structure
|
Common Name | H-1152 | ||
|---|---|---|---|---|
| CAS Number | 451462-58-1 | Molecular Weight | 319.42 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H21N3O2S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of H-1152H-1152 is a membrane-permeable and selective ROCK inhibitor, with a Ki value of 1.6 nM, and an IC50 value of 12 nM for ROCK2. |
| Name | 4-methyl-5-[(2-methyl-1,4-diazepan-1-yl)sulfonyl]isoquinoline,dihydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | H-1152 is a membrane-permeable and selective ROCK inhibitor, with a Ki value of 1.6 nM, and an IC50 value of 12 nM for ROCK2. |
|---|---|
| Related Catalog | |
| Target |
ROCKII:12 nM (IC50) CaMKII:0.18 μM (IC50) PKG:0.36 μM (IC50) AuroraA:0.745 μM (IC50) PKA:3.03 μM (IC50) Src:3.06 μM (IC50) PKC:5.68 μM (IC50) Abl:7.77 μM (IC50) MKK4:16.9 μM (IC50) MLCK:28.3 μM (IC50) EGFR:50 μM (IC50) GSK3α:60.7 μM (IC50) AMPK:100 μM (IC50) P38α:100 μM (IC50) |
| In Vitro | H-1152 is an inhibitor of Rho-kinase, with an IC50 of 12 nM for ROCK2. H-1152 (H-1152P) also shows less inhibitory activities against CaMKII, PKG, AuroraA, PKA, Src, PKC, MLCK, Abl, EGFR, MKK4, GSK3α, AMPK, and P38α, with IC50s of 0.180, 0.360, 0.745, 3.03, 3.06, 5.68, 28.3, 7.77, 50.0, 16.9, 60.7, 100, and 100 μM, respectively[1]. H-1152 potently inhibits Rho kinase, with a Ki of 1.6 nM, and slightly suppresses PKA, PKC and MLCK, with Kis of 0.63, 9.27, and 10.1 μM, respectively. H-1152 (0.1-10 µM) highly inhibits MARCKS phosphorylation, with an IC50 value of 2.5 µM in LPA-treated cells, but shows no such obvious effects in PDBu-treated cells[2]. H-1152 (0.5-10 μM) cuases no decreased neuronal survival. H-1152 (1, 5 or 10 μM) also exerts no alterations in the ratios of different neuronal morphologies. Furthermore, H-1152 (10 μM) increases neurite length in both BMP4 and LIF cultures[3]. |
| Kinase Assay | Inhibitors (including H-1152) are added at the indicated concentrations to 50 µL of the assay mixture 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 40 µM S6-peptide, various concentrations of [γ-32P]ATP and purified Rho-kinase. The reactions are started by the addition of [γ-32P]ATP and carried out at 30°C for 5 min. The Michaelis-Menten equation is used to calculate the Michaelis constant (Km) and maximal velocity (Vmax) of Rho-kinase. Data are further analyzed with secondary plot to calculate the inhibitory constant (Ki)[2]. |
| Cell Assay | Briefly, cells are routinely plated on poly-d-lysine/laminin coated 96 well plates or in 16 well glass culture slides. Control medium contained Dulbecco's modified Eagles medium/Hams F12(1:1) (DMEM/F12), 2 mM l-glutamine, N2 mix (1:100 dilution), 0.63 mL of 45% glucose for each 100 mL of DMEM/F12, neurotrophin 3 (NT3; final concentration, 8 ng/mL), BDNF (final concentration 8 ng/mL), and 10% fetal bovine serum heat inactivated before use. LIF cultures contain control medium+LIF (50 ng/mL). BMP4 cultures contain control medium+bone morphogenetic protein 4 (BMP4; 25 ng/mL). Total volume of culture is 110 μL. ROCK inhibitor H-1152 is diluted in water and added in an additional 10 μL to cultures 24 h after plating. Water is added to controls. Eighteen hours after the addition of inhibitor, cultures are fixed in 4% paraformaldehyde (1 h at room temperature for peroxidase-linked labeling and 20 min at room temperature for fluorescence labeling). For ArrayScan/Cellomics automated analysis: Cells are plated in a total volume of 50 μL on 384 well plastic plates previously coated with poly-d-lysine/laminin, and cultured in the same medium[3]. |
| References |
| Molecular Formula | C16H21N3O2S |
|---|---|
| Molecular Weight | 319.42 |
| PSA | 70.68000 |
| LogP | 4.86720 |
| Storage condition | 2-8℃ |
| 4-Methyl-5-{[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl}isoquinoline dihydrochloride |
| Isoquinoline, 5-[[(2S)-hexahydro-2-methyl-1H-1,4-diazepin-1-yl]sulfonyl]-4-methyl-, hydrochloride (1:2) |
| H-1152 |