Daucosterol
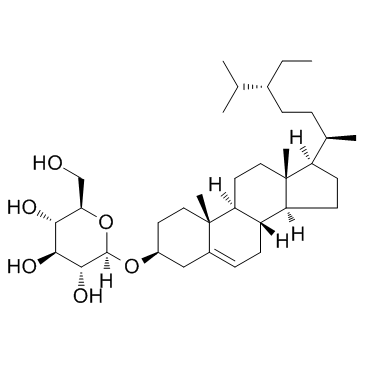
Daucosterol structure
|
Common Name | Daucosterol | ||
|---|---|---|---|---|
| CAS Number | 474-58-8 | Molecular Weight | 576.847 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 673.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C35H60O6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 361.2±31.5 °C | |
Use of DaucosterolDaucosterol is a natural sterolin. IC50 value:Target:In vitro: In the study of the effects of daucosterol on the survival of cultured cortical neurons after neurons were subjected to oxygen and glucose deprivation and simulated reperfusion (OGD/R)(2), the results showed that post-treatment of daucosterol significantly reduced neuronal loss, as well as apoptotic rate and caspase-3 activity, displaying the neuroprotective activity. We also found that daucosterol increased the expression level of IGF1 protein, diminished the down-regulation of p-AKT(3) and p-GSK-3β(4), thus activating the AKT(5) signal pathway [1]. Cell counting kit-8 (CCK-8) assay showed that daucosterol significantly increased the quantity of viable cells and the effectiveness of daucosterol was similar to that of basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) [2]. Daucosterol inhibits the proliferation of human breast cancer cell line MCF-7 and gastric cancer cell lines MGC803, BGC823 and AGS in a dose-dependent manner. Furthermore, daucosterol inhibits murine hepatoma H22 cell growth in ICR mice. Daucosterol treatment induces intracellular ROS generation and autophagy, but not apoptotic cell death. Treatment with ROS scavenger GSH (reduced glutathione), NAC (N-acetyl-l-cysteine) or autophagy inhibitor 3-Methyladenine (3-MA) counteracted daucosterol-induced autophagy and growth inhibition in BGC823 and MCF-7 cancer cells [3].In vivo: |
| Name | Daucosterol |
|---|---|
| Synonym | More Synonyms |
| Description | Daucosterol is a natural sterolin. IC50 value:Target:In vitro: In the study of the effects of daucosterol on the survival of cultured cortical neurons after neurons were subjected to oxygen and glucose deprivation and simulated reperfusion (OGD/R)(2), the results showed that post-treatment of daucosterol significantly reduced neuronal loss, as well as apoptotic rate and caspase-3 activity, displaying the neuroprotective activity. We also found that daucosterol increased the expression level of IGF1 protein, diminished the down-regulation of p-AKT(3) and p-GSK-3β(4), thus activating the AKT(5) signal pathway [1]. Cell counting kit-8 (CCK-8) assay showed that daucosterol significantly increased the quantity of viable cells and the effectiveness of daucosterol was similar to that of basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) [2]. Daucosterol inhibits the proliferation of human breast cancer cell line MCF-7 and gastric cancer cell lines MGC803, BGC823 and AGS in a dose-dependent manner. Furthermore, daucosterol inhibits murine hepatoma H22 cell growth in ICR mice. Daucosterol treatment induces intracellular ROS generation and autophagy, but not apoptotic cell death. Treatment with ROS scavenger GSH (reduced glutathione), NAC (N-acetyl-l-cysteine) or autophagy inhibitor 3-Methyladenine (3-MA) counteracted daucosterol-induced autophagy and growth inhibition in BGC823 and MCF-7 cancer cells [3].In vivo: |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 673.6±55.0 °C at 760 mmHg |
| Molecular Formula | C35H60O6 |
| Molecular Weight | 576.847 |
| Flash Point | 361.2±31.5 °C |
| Exact Mass | 576.438965 |
| PSA | 99.38000 |
| LogP | 8.78 |
| Vapour Pressure | 0.0±4.7 mmHg at 25°C |
| Index of Refraction | 1.554 |
| InChIKey | NPJICTMALKLTFW-OFUAXYCQSA-N |
| SMILES | CCC(CCC(C)C1CCC2C3CC=C4CC(OC5OC(CO)C(O)C(O)C5O)CCC4(C)C3CCC12C)C(C)C |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | 24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 29389090 |
| Precursor 7 | |
|---|---|
| DownStream 5 | |
|
[Chemical constituents contained in seeds of Notopterygium franchetii].
Zhongguo Zhong Yao Za Zhi 37(7) , 941-5, (2012) To study the chemical constituents from the seeds of Notopterygium franchetii.Ethanol extracts of seeds N. franchetii were separated and purified by such methods as normal and reversed phase column ch... |
|
|
[Study on the chemical constituets in ethyl acetante extraction from semen litchi].
Zhong Yao Cai 35(1) , 64-6, (2012) To study the chemical constituents in ethyl acetate extraction of Semen Litchi.The compounds were isolated and purified by column chromatography on silica gel and Sephadex LH-20 coupled with preparati... |
|
|
[Chemical constituents from stems of Dysoxylum laxiracemosum].
Zhongguo Zhong Yao Za Zhi 37(9) , 1237-40, (2012) Twelve compounds were separated from stems of Dysoxylum laxiracemosum and their structures were identified by spectrum analysis as shoreic acid (1), cabraleahydroxylactone (2), cabralealactone (3), ci... |
| β-D-Glucopyranoside, (3β)-stigmast-5-en-3-yl |
| Daucosterin |
| lyoniside |
| Coriandrinol |
| (3b)-Stigmast-5-en-3-yl b-D-Glucopyranoside |
| Sitogluside |
| β-Sitosterol β-D-glucoside |
| β-sitosterol-D-glucoside |
| b-Daucosterol |
| b-Sitosteryl glucoside |
| Alexandrin |
| Eleutheroside A |
| β-sitosterol 3-O-β-D-glucopyranoside |
| 1513DMIa |
| Sterolin |
| β-sitosteryl-β-D-glucopyranoside |
| 3b-(b-D-glucopyranosyloxy)stigmast-5-ene |
| Doursterol |
| (3β)-Stigmast-5-en-3-yl β-D-glucopyranoside |
| 3β-(β-D-Glucopyranosyloxy)stigmast-5-ene |
| Stigmast-5-ene, 3-β-(β-D-glucopyranosyloxy)- |
| BSSG |
| β-Sitosteryl glucoside |
| 3-β-D-glucosylsitosterol |
| 3-β-(β-D-Glucopyranosyloxy)stigmast-5-ene |
![3-[(2,3,4,6-tetra-O-benzyl-β-D-glucopyranosyl)oxy]-sitosterol Structure](https://image.chemsrc.com/caspic/093/137909-13-8.png) CAS#:137909-13-8
CAS#:137909-13-8 CAS#:83-46-5
CAS#:83-46-5 CAS#:572-09-8
CAS#:572-09-8 CAS#:4291-69-4
CAS#:4291-69-4 CAS#:74808-09-6
CAS#:74808-09-6 CAS#:53657-29-7
CAS#:53657-29-7 CAS#:18749-71-8
CAS#:18749-71-8 CAS#:2280-44-6
CAS#:2280-44-6 CAS#:50-99-7
CAS#:50-99-7 CAS#:1139-97-5
CAS#:1139-97-5
