Beta-Sitosterol
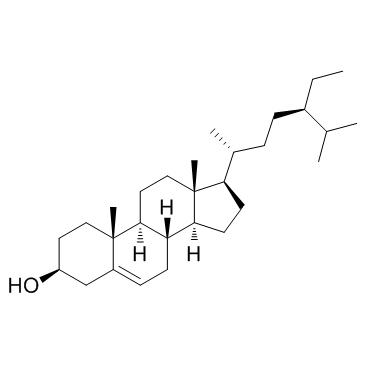
Beta-Sitosterol structure
|
Common Name | Beta-Sitosterol | ||
|---|---|---|---|---|
| CAS Number | 83-46-5 | Molecular Weight | 414.707 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 501.9±19.0 °C at 760 mmHg | |
| Molecular Formula | C29H50O | Melting Point | 139-142 ºC | |
| MSDS | Chinese USA | Flash Point | 220.4±13.7 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of Beta-SitosterolBeta-Sitosterol weakly inhibits porcine pancreatic lipase (PPL) activity. Sitosterol is an important compound extracted from the leaves of Aloe vera. |
| Name | sitosterol |
|---|---|
| Synonym | More Synonyms |
| Description | Beta-Sitosterol weakly inhibits porcine pancreatic lipase (PPL) activity. Sitosterol is an important compound extracted from the leaves of Aloe vera. |
|---|---|
| Related Catalog | |
| Target |
IC50: 99.99±1.86 μg/mL (PPL)[1] |
| In Vitro | Bioactivity-guided isolation afforded three compounds from the hexane fraction of E. indica, namely, Beta-Sitosterol (β-sitosterol), Stigmasterol, and Lutein. Both compounds are found to possess very low PPL inhibition activity, that is, 2.99±0.80% (Beta-Sitosterol) of inhibition at 100 μg/mL (242 μM) and 2.68±0.38% (Stigmasterol) of inhibition at 100 μg/mL (243 μM), respectively. Weak PPL inhibition activity of Beta-Sitosterol and Stigmasterol isolated from Alpinia zerumbet with IC50 value of 99.99±1.86 μg/mL and 125.05±4.76 μg/mL, respectively, in comparison with the inhibition shown by Curcumin (IC50=4.92±0.21 μg/mL) and Quercetin (IC50=18.60±0.86 μg/mL) which are used as positive controls in their study. Beta-Sitosterol and Stigmasterol are recorded with weak PPL inhibitory activity of only 3.0±0.8% and 2.7±0.4% at 100 μg/mL, respectively, (i.e., 242 μM and 243 μM) in contrast (34.5±5.4% at 100 μg/mL), which are comparatively lower than that recorded in literature (i.e., 50% PPL inhibition at 100 μg/mL)[1]. Sitosterol is an important compound extracted from the leaves of Aloe vera. It inhibits the growth of promastigotes of L. donovani, a causative agent for life threatening visceral leishmaniasis disease[2]. |
| In Vivo | Beta-Sitosterol (β-sitosterol) treatment significantly reduced the immobility time at three doses (10, 20, and 30 mg/kg) in the Forced Swim Test (FST) and Tail Suspension Test (TST), indicating an antidepressant effect. This effect is similar to the positive control fluoxetine (20 mg/kg) at a dose of 30 mg/kg, where the strongest effect is observed compared with the control group (P < 0.001). The same effects are observed for three doses of Beta-Sitosterol in the TST. The % DID values are as follows: FST: 39.27% (10 mg/kg), 51.23% (20 mg/kg), and 57.48% (30 mg/kg); TST: 31.63% (10 mg/kg), 43.95% (20 mg/kg), and 53.38% (30 mg/kg). These results indicate that Beta-Sitosterol has a significant antidepressant activity in mice during the FST and TST. Furthermore, Beta-Sitosterol exhibits the antidepressant effect in a dose-dependent manner[3]. |
| Animal Admin | Mice[3] Male ICR mice (20±2 g) and male KunMing mice (20±2 g) are used. Local breed, male ICR mice (20±2 g) are used in the FST under standard conditions with free access to food and water. Mice are randomly divided into four groups (8 mice per group are used) for the tail suspension test (TST): Beta-Sitosterol (10, 20, and 30 mg/kg), total sterols (50, 100, and 200 mg/kg), fluoxetine (20 mg/kg), or distilled water. 80 male mice are used. Briefly, the vehicle or test drugs are administered 30 min before a test session acute ip injection. Then, mice are individually suspended by tail with clamp (2 cm from the tip of the end) in a box (25 cm×25 cm×30 cm) with the head 5 cm to the bottom. Testing is carried out in a darkened room with minimal background noise. All animals are suspended for total 6 min, and the duration of immobility is observed and measured during the final 4-min interval of the test. All test sessions are recorded by a video camera positioned directly above the box. Two competent observers blind to treatment scored the videotapes. Mice consider immobile only when they hung passively and completely motionless. The animals are used only once in this test. All TSTs are performed between 11:00 A.M. and 14:00 P.M. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 501.9±19.0 °C at 760 mmHg |
| Melting Point | 139-142 ºC |
| Molecular Formula | C29H50O |
| Molecular Weight | 414.707 |
| Flash Point | 220.4±13.7 °C |
| Exact Mass | 414.386169 |
| PSA | 20.23000 |
| LogP | 10.73 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.521 |
| InChIKey | KZJWDPNRJALLNS-VJSFXXLFSA-N |
| SMILES | CCC(CCC(C)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C)C(C)C |
| Storage condition | -20°C |
| Water Solubility | INSOLUBLE |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H319-H331-H336-H351-H361d-H372 |
| Precautionary Statements | P201-P261-P304 + P340 + P312-P305 + P351 + P338-P308 + P313-P403 + P233 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xn |
| Risk Phrases | R38 |
| Safety Phrases | S22-S24/25-S36 |
| RIDADR | UN 1888 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | WJ2600000 |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Nutritional evaluation and health promoting activities of nuts and seeds cultivated in Greece.
Int. J. Food Sci. Nutr. 64(6) , 757-67, (2013) Available data suggest that genetic as well as environmental factors may influence nuts and seeds nutrients content. In this context nuts and seeds cultivated in Greece were studied. Macronutrients co... |
|
|
Elevated serum squalene and cholesterol synthesis markers in pregnant obese women with gestational diabetes mellitus.
J. Lipid Res. 55(12) , 2644-54, (2014) We examined serum cholesterol synthesis and absorption markers and their association with neonatal birth weight in obese pregnancies affected by gestational diabetes mellitus (GDM). Pregnant women at ... |
| Prostasal |
| L E5 B666 LUTJ A1 E1 FY1&2Y2&Y1&1 OQ &&Stereoisomer |
| Sitosterol β |
| B-Sitosterol |
| 22:23-Dihydrostigmasterol |
| 24b-Ethyl-D5-cholesten-3b-ol |
| Sitosterol |
| (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-éthyl-6-méthyl-2-heptanyl]-10,13-diméthyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tétradécahydro-1H-cyclopenta[a]phénanthrén-3-ol |
| (3β)-Stigmast-5-en-3-ol |
| Stigmast-5-en-3b-ol |
| (3b)-Stigmast-5-en-3-ol |
| α-Phytosterol |
| MFCD00003631 |
| (3β,20R,24R)-Stigmast-5-en-3-ol |
| β-Sitosterol |
| EINECS 201-480-6 |
| Sitosterin |
| a-Phytosterol |
| D5-Stigmasten-3b-ol |
| δ5-Stigmasten-3b-ol |
| b-Sitosterin |
| (24R)-Stigmast-5-en-3b-ol |
| a-Dihydrofucosterol |
| beta-Sitosterol |
| (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-Ethyl-6-methyl-2-heptanyl]-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| α-Dihydrofucosterol |
 CAS#:915-05-9
CAS#:915-05-9 CAS#:83-48-7
CAS#:83-48-7 CAS#:474-58-8
CAS#:474-58-8 CAS#:108646-29-3
CAS#:108646-29-3 CAS#:4651-48-3
CAS#:4651-48-3 CAS#:53603-94-4
CAS#:53603-94-4 CAS#:83787-28-4
CAS#:83787-28-4 CAS#:70813-67-1
CAS#:70813-67-1 CAS#:70813-69-3
CAS#:70813-69-3 CAS#:481-30-1
CAS#:481-30-1 CAS#:66252-74-2
CAS#:66252-74-2 CAS#:25694-37-5
CAS#:25694-37-5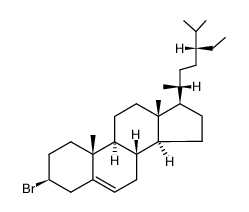 CAS#:66252-73-1
CAS#:66252-73-1 CAS#:18749-71-8
CAS#:18749-71-8 CAS#:58-22-0
CAS#:58-22-0
