Cepharanthine
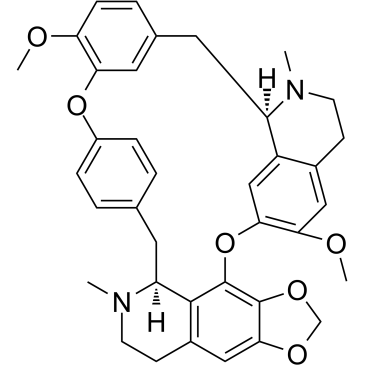
Cepharanthine structure
|
Common Name | Cepharanthine | ||
|---|---|---|---|---|
| CAS Number | 481-49-2 | Molecular Weight | 606.707 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C37H38N2O6 | Melting Point | 140 - 145ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CepharanthineCepharanthine, an alkaloid derived from Stephania cepharantha Hayata, with possesses anti-inflammatory and antioxidative activities[1][2][3]. Cepharanthine attenuates muscle and kidney injuries induced by limb ischemia/reperfusion (I/R)[3]. Cepharanthine induces autophagy, apoptosis and cell cycle arrest in breast cancer cells[4]. Cepharanthine inhibits the HIV-1 entry process by reducing plasma membrane fluidity[5]. |
| Name | Cepharanthine |
|---|---|
| Synonym | More Synonyms |
| Description | Cepharanthine, an alkaloid derived from Stephania cepharantha Hayata, with possesses anti-inflammatory and antioxidative activities[1][2][3]. Cepharanthine attenuates muscle and kidney injuries induced by limb ischemia/reperfusion (I/R)[3]. Cepharanthine induces autophagy, apoptosis and cell cycle arrest in breast cancer cells[4]. Cepharanthine inhibits the HIV-1 entry process by reducing plasma membrane fluidity[5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 140 - 145ºC |
| Molecular Formula | C37H38N2O6 |
| Molecular Weight | 606.707 |
| Exact Mass | 606.273010 |
| PSA | 61.86000 |
| LogP | 5.23 |
| Index of Refraction | 1.608 |
| InChIKey | YVPXVXANRNDGTA-WDYNHAJCSA-N |
| SMILES | COc1ccc2cc1Oc1ccc(cc1)CC1c3c(cc4c(c3Oc3cc5c(cc3OC)CCN(C)C5C2)OCO4)CCN1C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | T+ |
| RIDADR | NONH for all modes of transport |
| RTECS | FK0527000 |
|
Interaction of cepharanthine with immobilized heat shock protein 90α (Hsp90α) and screening of Hsp90α inhibitors.
Anal. Biochem. 434(1) , 202-6, (2013) Heat shock protein 90α (Hsp90α) immobilized on aminopropyl silica gels was prepared via the N- or C-terminal, which was termed Hsp90α-NT or Hsp90α-CT, respectively. Binding interactions of biscoclauri... |
|
|
Cepharanthine inhibited HIV-1 cell-cell transmission and cell-free infection via modification of cell membrane fluidity.
Bioorg. Med. Chem. Lett. 24(9) , 2115-7, (2014) The anti-HIV-1 activity of cepharanthine (CEP), a natural product derived from Stephania cepharantha Hayata, was evaluated. CEP stabilized plasma membrane fluidity and inhibited HIV-1 envelope-depende... |
|
|
Recent advances regarding the role of ABC subfamily C member 10 (ABCC10) in the efflux of antitumor drugs.
Chin. J. Cancer 33(5) , 223-30, (2014) ABCC10, also known as multidrug-resistant protein 7 (MRP7), is the tenth member of the C subfamily of the ATP-binding cassette (ABC) superfamily. ABCC10 mediates multidrug resistance (MDR) in cancer c... |
| O-Methylcepharanoline |
| (14S,27R)-22,33-Dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.2.1.1.0.0.0]nonatriaconta-1(33),3,8,10(39),16,18,21(36),22,24,31,34,37-dod ecaene |
| 6',12'-dimethoxy-2,2'-dimethyl-6,7-[methylenebis-(oxy)]oxyacanthan |
| CEPHARANTHINE (RG) |
| (+)-cepharanthine |
| [methylenebis(oxy)] |
| Cepharanthine |
| sepharanthine |
| Cepharantin |
| CEPHARANTHINUM |
| (14S,27R)-22,33-Dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.2.1.1.0.0.0]nonatriaconta-1(33),3,8,10(39),16,18,21(36),22,24,31,34,37-dodecaene |
| 6',12'-Dimethoxy-2,2'-dimethyl-6,7-[methylenebis(oxy)]oxyacanthan |
| 6,7-methanediyldioxy-6',12'-dimethoxy-2,2'-dimethyl-oxyacanthane |
| Stephanotis |
| Cepharanthin |


