CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DK1750000
-
CHEMICAL NAME :
-
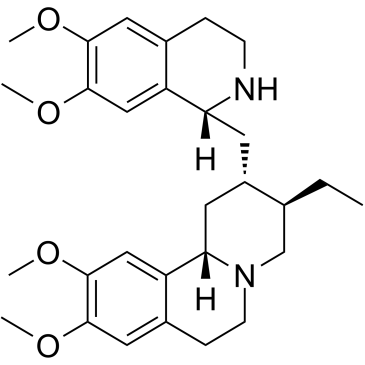
2H-Benzo(a)quinolizine, 3-ethyl-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-2-((1, 2,3,4- tetrahydro-6,7-dimethoxy-1-isoquinolyl)methyl)-
-
CAS REGISTRY NUMBER :
-
483-18-1
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
18
-
MOLECULAR FORMULA :
-
C29-H40-N2-O4
-
MOLECULAR WEIGHT :
-
480.71
-
WISWESSER LINE NOTATION :
-
T B666 GNTT&J E2 LO1 MO1 D1- BT66 CMT&J HO1 IO1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Rinsed with water
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration into the eye
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
10 mg/kg/10D
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Cardiac - arrhythmias (including changes in conduction) Gastrointestinal - other changes
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
2941 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
12 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
12 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
8 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
30 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
2 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
70 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
7 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - mouse
-
DOSE/DURATION :
-
25 mg/kg
-
REFERENCE :
-
JNCIAM Journal of the National Cancer Institute. (Washington, DC) V.1-60, 1940-78. For publisher information, see JJIND8. Volume(issue)/page/year: 60,1049,1978 *** REVIEWS *** TOXICOLOGY REVIEW CRTXB2 CRC Critical Reviews in Toxicology. (CRC Press, Inc., 2000 Corporate Blvd., NW, Boca Raton, FL 33431) V.1- 1971- Volume(issue)/page/year: 2,159,1973 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4638 No. of Facilities: 7 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 103 (estimated) No. of Female Employees: 52 (estimated)
|
 CAS#:1609-47-8
CAS#:1609-47-8 CAS#:105-58-8
CAS#:105-58-8 CAS#:90-05-1
CAS#:90-05-1 CAS#:75-50-3
CAS#:75-50-3 CAS#:577-68-4
CAS#:577-68-4