Allocryptopine
Modify Date: 2024-01-02 22:11:10
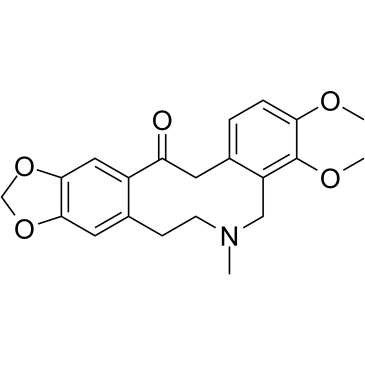
Allocryptopine structure
|
Common Name | Allocryptopine | ||
|---|---|---|---|---|
| CAS Number | 485-91-6 | Molecular Weight | 369.411 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 552.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H23NO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 288.1±30.1 °C | |
Use of AllocryptopineAllocryptopine, a derivative of tetrahydropalmatine, is extracted from Corydalis decumbens (Thunb.) Pers. Papaveraceae. Allocryptopine has antiarrhythmic effects and potently blocks human ether-a-go-go related gene (hERG) current[1][2]. |
| Name | allocryptopine |
|---|---|
| Synonym | More Synonyms |
| Description | Allocryptopine, a derivative of tetrahydropalmatine, is extracted from Corydalis decumbens (Thunb.) Pers. Papaveraceae. Allocryptopine has antiarrhythmic effects and potently blocks human ether-a-go-go related gene (hERG) current[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 552.7±50.0 °C at 760 mmHg |
| Molecular Formula | C21H23NO5 |
| Molecular Weight | 369.411 |
| Flash Point | 288.1±30.1 °C |
| Exact Mass | 369.157623 |
| PSA | 57.23000 |
| LogP | 3.64 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.574 |
| Storage condition | 2-8C |
| Precursor 7 | |
|---|---|
| DownStream 2 | |
| allo-Cryptopine |
| Allocryptopine |
| β-Homochelidonine |
| Benzo[c][1,3]benzodioxolo[5,6-g]azecin-14(6H)-one, 5,7,8,15-tetrahydro-3,4-dimethoxy-6-methyl- |
| [1,3]Benzodioxolo[5,6-e][2]benzazecin-14(6H)-one, 5,7,8,15-tetrahydro-3,4-dimethoxy-6-methyl- |
| 5,7,8,15-Tetrahydro-3,4-dimethoxy-6-methylbenzo[e][1,3]dioxolo[4,5-k][3]benzazecin-14(6H)-one |
| 5,7,8,15-Tetrahydro-3,4-dimethoxy-6-methylbenzo(e)(1,3)dioxolo(4,5-k)(3)benzazecin-14(6H)-one |
| Thalictrimine |
| Fagarine I |
| a-allo-Cryptopine |
| Benzo(e)(1,3)dioxolo(4,5-k)(3)benzazecin-14(6H)-one, 5,7,8,15-tetrahydro-3,4-dimethoxy-6-methyl- |
| 3,4-Dimethoxy-6-methyl-5,7,8,15-tetrahydrobenzo[c][1,3]benzodioxolo[5,6-g]azecin-14(6H)-one |
| α-Fagarine |
![6H-Benzo[g]-1,3-benzodioxolo[5,6-a]quinolizine,5,8,13,13a-tetrahydro-9,10-dimethoxy- Structure](https://image.chemsrc.com/caspic/313/29074-38-2.png) CAS#:29074-38-2
CAS#:29074-38-2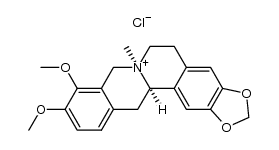 CAS#:16211-68-0
CAS#:16211-68-0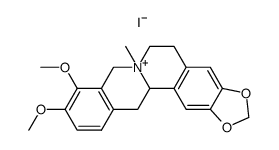 CAS#:61774-67-2
CAS#:61774-67-2![3,4-dimethoxy-6-methyl-5,6,7,8-tetrahydro-11H-benzo[c][1,3]benzodioxolo[5,6-g]azecine Structure](https://image.chemsrc.com/caspic/298/41759-47-1.png) CAS#:41759-47-1
CAS#:41759-47-1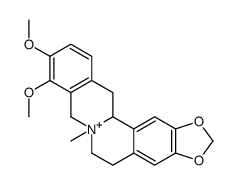 CAS#:47474-49-7
CAS#:47474-49-7![3,4-dimethoxy-6-methyl-5,6,7,8-tetrahydro-benzo[c][1,3]dioxolo[4',5':4,5]benz[1,2-g]azecine-6-oxide Structure](https://image.chemsrc.com/caspic/260/41759-48-2.png) CAS#:41759-48-2
CAS#:41759-48-2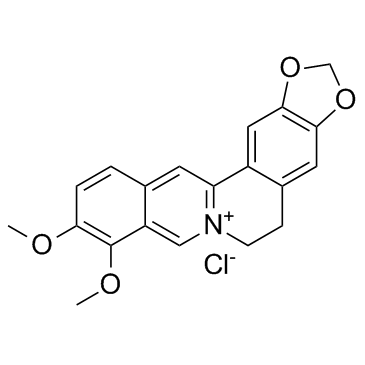 CAS#:633-65-8
CAS#:633-65-8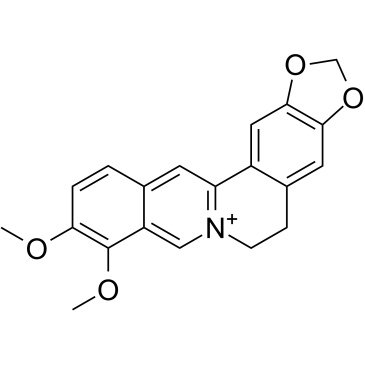 CAS#:2086-83-1
CAS#:2086-83-1 CAS#:6880-91-7
CAS#:6880-91-7
