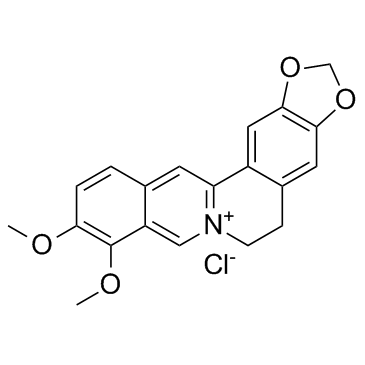
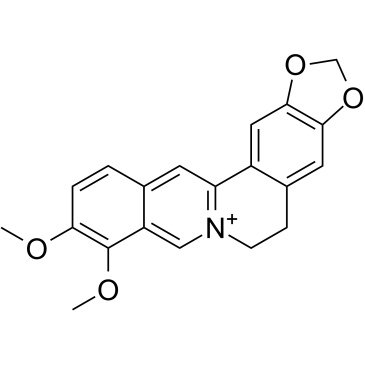
485-91-6
| Name | allocryptopine |
|---|---|
| Synonyms |
allo-Cryptopine
Allocryptopine β-Homochelidonine 5,7,8,15-Tetrahydro-3,4-dimethoxy-6-methylbenzo[e][1,3]dioxolo[4,5-k][3]benzazecin-14(6H)-one 5,7,8,15-Tetrahydro-3,4-dimethoxy-6-methylbenzo(e)(1,3)dioxolo(4,5-k)(3)benzazecin-14(6H)-one Thalictrimine Fagarine I a-allo-Cryptopine 3,4-Dimethoxy-6-methyl-5,7,8,15-tetrahydrobenzo[c][1,3]benzodioxolo[5,6-g]azecin-14(6H)-one α-Fagarine |
| Description | Allocryptopine, a derivative of tetrahydropalmatine, is extracted from Corydalis decumbens (Thunb.) Pers. Papaveraceae. Allocryptopine has antiarrhythmic effects and potently blocks human ether-a-go-go related gene (hERG) current[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 552.7±50.0 °C at 760 mmHg |
| Molecular Formula | C21H23NO5 |
| Molecular Weight | 369.411 |
| Flash Point | 288.1±30.1 °C |
| Exact Mass | 369.157623 |
| PSA | 57.23000 |
| LogP | 3.64 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.574 |
| Storage condition | 2-8C |
| Precursor 7 | |
|---|---|
| DownStream 2 | |


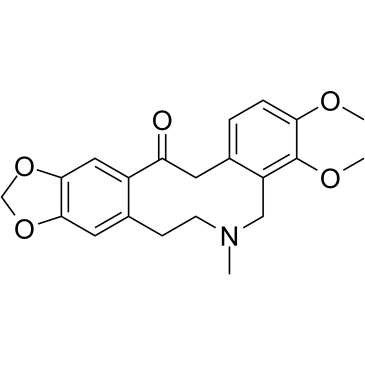
![6H-Benzo[g]-1,3-benzodioxolo[5,6-a]quinolizine,5,8,13,13a-tetrahydro-9,10-dimethoxy structure](https://image.chemsrc.com/caspic/313/29074-38-2.png)
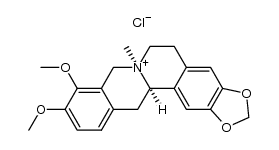
![3,4-dimethoxy-6-methyl-5,6,7,8-tetrahydro-11H-benzo[c][1,3]benzodioxolo[5,6-g]azecine structure](https://image.chemsrc.com/caspic/298/41759-47-1.png)
![3,4-dimethoxy-6-methyl-5,6,7,8-tetrahydro-benzo[c][1,3]dioxolo[4',5':4,5]benz[1,2-g]azecine-6-oxide structure](https://image.chemsrc.com/caspic/260/41759-48-2.png)