Canthaxanthin
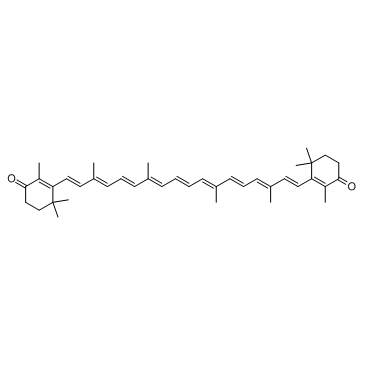
Canthaxanthin structure
|
Common Name | Canthaxanthin | ||
|---|---|---|---|---|
| CAS Number | 514-78-3 | Molecular Weight | 564.840 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 717.0±40.0 °C at 760 mmHg | |
| Molecular Formula | C40H52O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 253.9±24.3 °C | |
Use of CanthaxanthinCanthaxanthin is a red-orange carotenoid with various biological activities, such as antioxidant, antitumor properties. |
| Name | canthaxanthin |
|---|---|
| Synonym | More Synonyms |
| Description | Canthaxanthin is a red-orange carotenoid with various biological activities, such as antioxidant, antitumor properties. |
|---|---|
| Related Catalog | |
| In Vitro | Canthaxanthin enrichment of LDL has the potential to protect cholesterol from oxidation. In addition to its free radical scavenging and antioxidant properties (e.g., the induction of catalase and superoxide dismutase), canthaxanthin shows immunomodulatory activity (e.g. enhancing the proliferation and function of immune competent cells) and plays important role in gap junction communication (e.g. induction of the transmembrane protein connexin)[1]. At concentrations of 0.1 to 1 x 1000 μM, canthaxanthin significantly reduces the overall number of tumor cells. The greatest inhibition is observed at a canthaxanthin concentration of 1000 after 72 h and 96 h of incubation[2]. |
| In Vivo | Canthaxanthin alters the protective ability of tissues against oxidative stress. Canthaxanthin treatment for 15 d at the dose of 14 μg/kg body weight results in hepatic incorporation of the carotenoid, which is maximum in liver and reaches 0.52 ± 0.05 nmol/g liver. Glutathione peroxidase activity is 35% lower and catalase (59%) and manganese superoxide dismutase (28%) activities are higher in canthaxanthin-treated mice than in controls[3]. Canthaxanthin inhibit the growth of mammary tumors in mice and the anti-tumor activity is also influenced by the supplemental dose[4]. Diet supplementation with canthaxanthin for 3 weeks prior to the carcinogen results in a 65% reduction in the number of mammary cancers by a mechanism not involving pro-vitamin A activity[5]. |
| Animal Admin | Mice: Female 6-wk-old Balb/c mice are randomly divided into two groups (n = 10/group). The control group receives olive oil alone (vehicle) and the canthaxanthin-treated group receives canthaxanthin at a dose of 14 μg/kg body weight per day. At the end of the treatment, mice are killed by cervical dislocation and liver is excised, frozen in liquid nitrogen and stored at −80°C[3]. |
| References |
[1]. Esatbeyoglu T, et al. Canthaxanthin: From molecule to function. Mol Nutr Food Res. 2017 Jun;61(6). |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 717.0±40.0 °C at 760 mmHg |
| Molecular Formula | C40H52O2 |
| Molecular Weight | 564.840 |
| Flash Point | 253.9±24.3 °C |
| Exact Mass | 564.396729 |
| PSA | 34.14000 |
| LogP | 10.69 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.575 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Risk Phrases | 25-36/37/38 |
| Safety Phrases | 45-36/37/39-28A-26 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | GA0875000 |
| Packaging Group | II |
| Hazard Class | 6.1 |
| HS Code | 3204199000 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 3204199000 |
|---|
|
Developing an emulsion model system containing canthaxanthin biosynthesized by Dietzia natronolimnaea HS-1.
Int. J. Biol. Macromol. 51(4) , 618-26, (2012) An acceptable strategy to incorporate canthaxanthin (CX) as a natural colorant into products is by means of oil-in-water emulsions. The used CX in this study was produced by bacterium Dietzia natronol... |
|
|
Influence of canthaxanthin on broiler breeder reproduction, chick quality, and performance.
Poult. Sci. 90(7) , 1516-22, (2011) To investigate the effect of canthaxanthin supplied via a maternal route on the production of both breeder hens and chickens, 270 Chinese Three-Yellow breeder hens were randomly divided into 2 groups ... |
|
|
Determination of supplemental feeding needs for astaxanthin and canthaxanthin in salmonids by supramolecular solvent-based microextraction and liquid chromatography-UV/VIS spectroscopy.
Food Chem. 134(2) , 1244-9, (2012) Development of simple and rapid analytical methods for predicting supplemental feeding requirements in aquaculture is a need to reduce production costs. In this article, a supramolecular solvent (SUPR... |
|
Name: ERK5 transcriptional activity HTS
Source: 24565
Target: N/A
External Id: ERK5 transcriptional activity-HTS
|
|
Name: qHTS assay to identify small molecule antagonists of the androgen receptor (AR) signa...
Source: 824
Target: AR protein [Homo sapiens]
External Id: MDAN535
|
|
Name: qHTS for Inhibitors of human tyrosyl-DNA phosphodiesterase 1 (TDP1): qHTS in cells in...
Source: NCGC
Target: TDP1 protein [Homo sapiens]
External Id: TDP1100
|
|
Name: qHTS for Inhibitors of human tyrosyl-DNA phosphodiesterase 1 (TDP1): qHTS in cells in...
Source: NCGC
Target: TDP1 protein [Homo sapiens]
External Id: TDP1101
|
|
Name: Rescue cell viability in cybrid cells with a genetic mutation in complex 1 of the mit...
Source: ICCB-Longwood/NSRB Screening Facility, Harvard Medical School
Target: N/A
External Id: HMS1315
|
|
Name: qHTS assay to identify small molecule antagonists of the androgen receptor (AR) sign...
Source: 824
Target: N/A
External Id: MDAV150
|
|
Name: A screen for compounds that inhibit the activity of LtaS in Staphylococcus aureus
Source: ICCB-Longwood/NSRB Screening Facility, Harvard Medical School
External Id: HMS979
|
|
Name: Phenotypic Assay to Identify Small Molecules that Upregulate Production of hCFTR in H...
Source: Southern Research Institute
Target: CFTR
External Id: CF Folding
|
|
Name: The chemical genetic matrix (CGM) dataset as reported in Wildenhain et al. (2015) Pre...
Source: 11924
Target: N/A
External Id: CGM data for Cell Systems paper Dec 2015
|
|
Name: qHTS assay for small molecules that induce genotoxicity in human embryonic kidney cel...
Source: 824
Target: RecName: Full=ATPase family AAA domain-containing protein 5; AltName: Full=Chromosome fragility-associated gene 1 protein
External Id: ELG271
|
| MFCD00016364 |
| (7cis,9cis,11cis,13cis)-β,β-Carotene-4,4'-dione |
| Canthaxanthin |
| all-trans-Canthaxanthin |
| EINECS 208-187-2 |
 CAS#:7235-40-7
CAS#:7235-40-7 CAS#:29065-03-0
CAS#:29065-03-0 CAS#:5056-17-7
CAS#:5056-17-7![(2E,4E)-[5-(2,6,6-trimethyl-3-oxo-1-cyclohexen-1-yl)-3-methyl-2,4-pentadien-1-yl]triphenylphosphonium bromide Structure](https://image.chemsrc.com/caspic/138/63184-93-0.png) CAS#:63184-93-0
CAS#:63184-93-0 CAS#:432-68-8
CAS#:432-68-8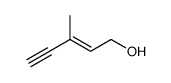 CAS#:6153-06-6
CAS#:6153-06-6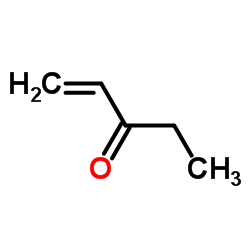 CAS#:1629-58-9
CAS#:1629-58-9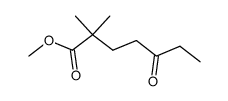 CAS#:64095-45-0
CAS#:64095-45-0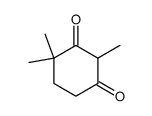 CAS#:63184-86-1
CAS#:63184-86-1 CAS#:63184-87-2
CAS#:63184-87-2