CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TU4725000
-
CHEMICAL NAME :
-
17-alpha-Pregn-4-ene-21-carboxylic acid, 17-hydroxy-7-alpha-mercapto-3-oxo-, gamma- lactone acetate
-
CAS REGISTRY NUMBER :
-
52-01-7
-
BEILSTEIN REFERENCE NO. :
-
0057767
-
LAST UPDATED :
-
199707
-
DATA ITEMS CITED :
-
37
-
MOLECULAR FORMULA :
-
C24-H32-O4-S
-
MOLECULAR WEIGHT :
-
416.62
-
WISWESSER LINE NOTATION :
-
L E5 B666 FX OV MUTJ A1 E1 KSV1 F-& CT5VOXTJ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
70 mg/kg/5W-I
-
TOXIC EFFECTS :
-
Blood - agranulocytosis
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 289,731,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
122 mg/kg/61D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis)
-
REFERENCE :
-
NZMJAX New Zealand Medical Journal. (New Zealand Medical Assoc., P.O. Box 156, Wellington, New Zealand) V.1- 1900- Volume(issue)/page/year: 83,147,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
5600 mg/kg
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume increased Nutritional and Gross Metabolic - changes in sodium Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
JLCMAK Journal of Laboratory and Clinical Medicine. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63141) V.1- 1915- Volume(issue)/page/year: 80,224,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
5714 ug/kg/4D-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - fibrosis, focal (pneumoconiosis) Blood - agranulocytosis Blood - changes in cell count (unspecified)
-
REFERENCE :
-
DICPBB Drug Intelligence and Clinical Pharmacy. (POB 42435, Cincinnati, OH 45242) V.3- 1969- Volume(issue)/page/year: 21,974,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,381,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
277 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,381,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JEPTDQ Journal of Environmental Pathology and Toxicology. (Park Forest South, IL) V.1-5(3), 1977-81(?). For publisher information, see JEPOEC. Volume(issue)/page/year: 1(5),641,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
260 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,381,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JEPTDQ Journal of Environmental Pathology and Toxicology. (Park Forest South, IL) V.1-5(3), 1977-81(?). For publisher information, see JEPOEC. Volume(issue)/page/year: 1(5),641,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
866 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JEPTDQ Journal of Environmental Pathology and Toxicology. (Park Forest South, IL) V.1-5(3), 1977-81(?). For publisher information, see JEPOEC. Volume(issue)/page/year: 1(5),641,1978 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4550 mg/kg/13W-C
-
TOXIC EFFECTS :
-
Endocrine - thyroid weight (goiter) Endocrine - changes in thyroid weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 98,263,1989 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1208 mg/kg
-
SEX/DURATION :
-
male 47 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - impotence Reproductive - Paternal Effects - breast development
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 2,729,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1692 mg/kg
-
SEX/DURATION :
-
female 47 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 2,729,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
28570 ug/kg
-
SEX/DURATION :
-
male 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - other effects on male
-
REFERENCE :
-
JCEMAZ Journal of Clinical Endocrinology and Metabolism. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.12- 1952- Volume(issue)/page/year: 41,777,1975
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
180 mg/kg
-
SEX/DURATION :
-
male 12 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - breast development Reproductive - Paternal Effects - other effects on male
-
REFERENCE :
-
CLREAS Clinical Research. (Slack Inc., 6900 Grove Rd., Thorofare, NJ 08086) V.6- 1958- Volume(issue)/page/year: 24,279A,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
252 mg/kg
-
SEX/DURATION :
-
female 12 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Maternal Effects - other effects
-
REFERENCE :
-
CLREAS Clinical Research. (Slack Inc., 6900 Grove Rd., Thorofare, NJ 08086) V.6- 1958- Volume(issue)/page/year: 24,279A,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
85700 ug/kg
-
SEX/DURATION :
-
male 60 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - impotence
-
REFERENCE :
-
JAMAAP JAMA, Journal of the American Medical Association. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1- 1883- Volume(issue)/page/year: 223,82,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
171 mg/kg
-
SEX/DURATION :
-
male 17 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - breast development
-
REFERENCE :
-
JAMAAP JAMA, Journal of the American Medical Association. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1- 1883- Volume(issue)/page/year: 223,82,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
280 mg/kg
-
SEX/DURATION :
-
female 35 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Maternal Effects - breasts, lactation (prior to or during pregnancy)
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 69,685,1968
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
male 35 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - impotence Reproductive - Paternal Effects - breast development
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 69,685,1968
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 30 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
REFERENCE :
-
JAMAAP JAMA, Journal of the American Medical Association. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1- 1883- Volume(issue)/page/year: 211,2014,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 9-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - abortion
-
REFERENCE :
-
FESTAS Fertility and Sterility. (American Fertility Soc., 608 13th Ave. S, Birmingham, AL 35282) V.1- 1950- Volume(issue)/page/year: 22,735,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
360 mg/kg
-
SEX/DURATION :
-
female 13-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
ACENA7 Acta Endocrinologica (Copenhagen). (Periodica, Skolegade 12 E, DK-2500 Valby, Denmark) V.1- 1948- Volume(issue)/page/year: 95,540,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 35,459,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2500 mg/kg
-
SEX/DURATION :
-
female 25 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes Reproductive - Maternal Effects - other effects
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 66,221,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
700 mg/kg
-
SEX/DURATION :
-
female 7 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 66,221,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
40 mg/kg
-
SEX/DURATION :
-
male 4 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
REFERENCE :
-
BIREBV Biology of Reproduction. (Soc. for the Study of Reproduction, 309 W. Clark St., Champaign, IL 61820) V.1- 1969- Volume(issue)/page/year: 27,771,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
70 mg/kg
-
SEX/DURATION :
-
female 14-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
BIREBV Biology of Reproduction. (Soc. for the Study of Reproduction, 309 W. Clark St., Champaign, IL 61820) V.1- 1969- Volume(issue)/page/year: 32,1051,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
160 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 35,459,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2 gm/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating female 1-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated) Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 66,221,1982 *** REVIEWS *** IARC Cancer Review:Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 24,259,1980 IARC Cancer Review:Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 24,259,1980 IARC Cancer Review:Group 3 IMSUDL IARC Monographs, Supplement. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) No.1- 1979- Volume(issue)/page/year: 7,344,1987 TOXICOLOGY REVIEW AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 85,630,1976 TOXICOLOGY REVIEW BIREBV Biology of Reproduction. (Soc. for the Study of Reproduction, 309 W. Clark St., Champaign, IL 61820) V.1- 1969- Volume(issue)/page/year: 8,259,1973 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4892 No. of Facilities: 79 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 854 (estimated) No. of Female Employees: 446 (estimated)
|
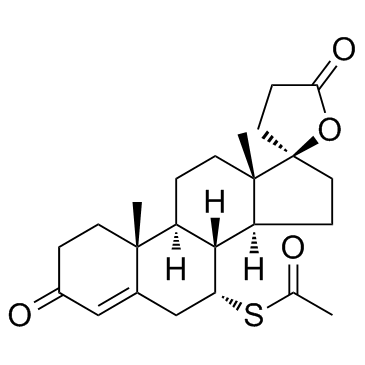


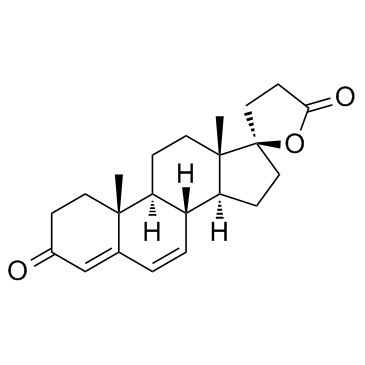

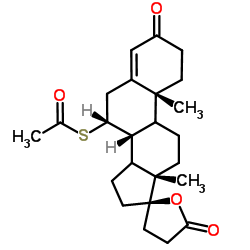


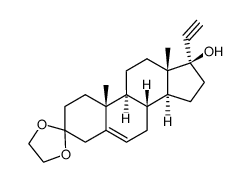
![5'-hydroxyspiro[pregnane-17,2'-tetrahydrofuran]-5-en-3-one structure](https://image.chemsrc.com/caspic/151/121936-43-4.png)

 CAS#:38753-77-4
CAS#:38753-77-4 CAS#:79327-11-0
CAS#:79327-11-0
