Quercitrin
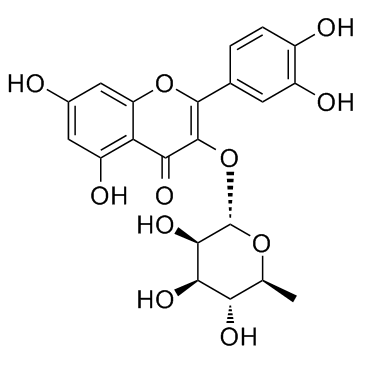
Quercitrin structure
|
Common Name | Quercitrin | ||
|---|---|---|---|---|
| CAS Number | 522-12-3 | Molecular Weight | 448.377 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 814.0±65.0 °C at 760 mmHg | |
| Molecular Formula | C21H20O11 | Melting Point | 174-183ºC | |
| MSDS | Chinese USA | Flash Point | 288.3±27.8 °C | |
Use of QuercitrinQuercitrin is a natural compound found in Tartary buckwheat with a potential anti-inflammation effect that is used to treat heart and vascular conditions.IC50 value:Target:In vitro: There were significant increases in caspase-3 activity, loss of MMP, and increases in the apoptotic cell population in response to quercitrin in DLD-1 colon cancer cells in a time- and dose-dependent manner. [1] In vivo: ICR mice received CCl4 intraperitoneally with or without quercitrin co-administration for 4 weeks. Data showed that quercitrin significantly suppressed the elevation of reactive oxygen species (ROS) production and malondialdehyde (MDA) content, reduced tissue plasminogen activator (t-PA) activity, enhanced the antioxidant enzyme activities and abrogated cytochrome P450 2E1 (CYP2E1) induction in mouse brains. [2] |
| Name | quercitrin |
|---|---|
| Synonym | More Synonyms |
| Description | Quercitrin is a natural compound found in Tartary buckwheat with a potential anti-inflammation effect that is used to treat heart and vascular conditions.IC50 value:Target:In vitro: There were significant increases in caspase-3 activity, loss of MMP, and increases in the apoptotic cell population in response to quercitrin in DLD-1 colon cancer cells in a time- and dose-dependent manner. [1] In vivo: ICR mice received CCl4 intraperitoneally with or without quercitrin co-administration for 4 weeks. Data showed that quercitrin significantly suppressed the elevation of reactive oxygen species (ROS) production and malondialdehyde (MDA) content, reduced tissue plasminogen activator (t-PA) activity, enhanced the antioxidant enzyme activities and abrogated cytochrome P450 2E1 (CYP2E1) induction in mouse brains. [2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 814.0±65.0 °C at 760 mmHg |
| Melting Point | 174-183ºC |
| Molecular Formula | C21H20O11 |
| Molecular Weight | 448.377 |
| Flash Point | 288.3±27.8 °C |
| Exact Mass | 448.100555 |
| PSA | 190.28000 |
| LogP | 2.17 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.776 |
| InChIKey | OXGUCUVFOIWWQJ-HQBVPOQASA-N |
| SMILES | CC1OC(Oc2c(-c3ccc(O)c(O)c3)oc3cc(O)cc(O)c3c2=O)C(O)C(O)C1O |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UZ5950000 |
|
~% 
Quercitrin CAS#:522-12-3 |
| Literature: Isobe, Takahiko; Kanazawa, Keiji; Fujimura, Makoto; Noda, Yukinao Bulletin of the Chemical Society of Japan, 1981 , vol. 54, # 10 p. 3239 |
|
Separation of the antioxidant compound quercitrin from Lindera obtusiloba Blume and its antimelanogenic effect on B16F10 melanoma cells.
Biosci. Biotechnol. Biochem. 77(1) , 58-64, (2013) Considering the growing evidence of the presence of antioxidant compounds in plant extracts, the objectives of this study were to identify antioxidant compounds in Lindera obtusiloba Blume (Lauraceae)... |
|
|
[Chemical constituents from Commelina communis].
Zhongguo Zhong Yao Za Zhi 38(19) , 3304-8, (2013) To investigate the chemical constituents from Commelina communis, fifteen compounds were separated and purified by silica gel, Sephadex LH-20, and ODS column chromatography, and semi-preparative HPLC.... |
|
|
Analysis of different European hazelnut (Corylus avellana L.) cultivars: authentication, phenotypic features, and phenolic profiles.
J. Agric. Food Chem. 62(26) , 6236-46, (2014) Hazelnuts exhibit functional properties due to their content in fatty acids and phenolic compounds that could positively affect human health. The food industry requires precise traits for morphologica... |
|
Name: Luminescence-based cell-based primary high throughput screening assay to identify ago...
Source: The Scripps Research Institute Molecular Screening Center
Target: mu-type opioid receptor isoform MOR-1 [Homo sapiens]
External Id: OPRM1-OPRD1_AG_LUMI_1536_1X%ACT PRUN
|
|
Name: Fluorescence-based cell-based primary high throughput screening assay to identify ago...
Source: The Scripps Research Institute Molecular Screening Center
Target: muscarinic acetylcholine receptor M1 [Homo sapiens]
External Id: CHRM1_AG_FLUO8_1536_1X%ACT PRUN
|
|
Name: Inhibition of PTP1B (unknown origin) at 20 ug/ml using pNPP substrate measured after ...
Source: ChEMBL
Target: Tyrosine-protein phosphatase non-receptor type 1
External Id: CHEMBL3405964
|
|
Name: Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by co...
Source: ChEMBL
Target: Tyrosine-protein phosphatase non-receptor type 1
External Id: CHEMBL3405965
|
|
Name: Inhibition of HIV1 recombinant integrase expressed in Escherichia coli
Source: ChEMBL
Target: Integrase
External Id: CHEMBL1026710
|
|
Name: Fluorescence-based cell-based primary high throughput screening assay to identify pos...
Source: The Scripps Research Institute Molecular Screening Center
Target: muscarinic acetylcholine receptor M1 [Homo sapiens]
External Id: CHRM1_PAM_FLUO8_1536_1X%ACT PRUN
|
|
Name: Fluorescence polarization-based biochemical high throughput primary assay to identify...
Source: The Scripps Research Institute Molecular Screening Center
Target: RecName: Full=Sialate O-acetylesterase; AltName: Full=H-Lse; AltName: Full=Sialic acid-specific 9-O-acetylesterase; Flags: Precursor [Homo sapiens]
External Id: SIAE_INH_FP_1536_1X%INH PRUN
|
|
Name: MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Activator_SinglePoint...
Source: Broad Institute
Target: N/A
External Id: 2084-01_Activator_SinglePoint_HTS_Activity
|
|
Name: Counterscreen for inhibitors of the fructose-bisphosphate aldolase (FBA) of M. tuberc...
Source: The Scripps Research Institute Molecular Screening Center
Target: N/A
External Id: GDH-TPI_INH_ABS_1536_1X%INH CSRUN
|
|
Name: Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyd...
Source: ChEMBL
Target: Bifunctional epoxide hydrolase 2
External Id: CHEMBL4717560
|
| quercetin 3-O-α-L-rhamnopyranoside |
| quercetin-3-L-rhamnoside |
| 3-O-α-rhamnosylquercetine |
| Quercetin 3-O-L-rhamnoside |
| Quercitroside |
| QUERCETIN 3-L-RHAMNOSIDE |
| EINECS 208-322-5 |
| Thujin |
| 3,3',4',5,7-Pentahydroxyflavone 3-(6-deoxy-a-L-mannopyranoside) |
| 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-on |
| 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one |
| Quercetin 3-O-α-L-rhamnoside |
| 2-(3,4-Dihydroxyphényl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}-4H-chromén-4-one |
| Quercetin-3-O-α-L-rhamnopyranoside |
| 3-[(6-Deoxy-α-L-mannopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-benzopyran-4-one |
| Quercimelin |
| 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl-6-deoxy-α-L-mannopyranoside |
| Quercetin-3-rhamnoside |
| Quercetrin |
| FLAVIN |
| Quercetin-3-O-rhamnoside |
| 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 6-deoxy-α-L-mannopyranoside |
| usafcf-2 |
| quercetin 3-O-rhamnoside |
| MFCD00016932 |
| 3-[(6-Deoxy-a-L-mannopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one |
| Quercitrin |
| quercetin 3-rhamnoside |
 CAS#:117-39-5
CAS#:117-39-5 CAS#:6014-42-2
CAS#:6014-42-2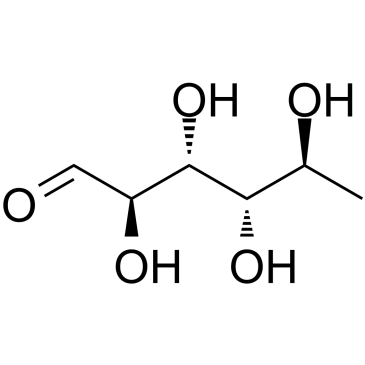 CAS#:3615-41-6
CAS#:3615-41-6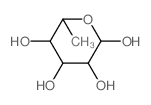 CAS#:73-34-7
CAS#:73-34-7
