522-12-3
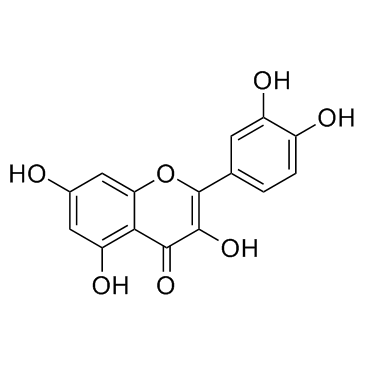
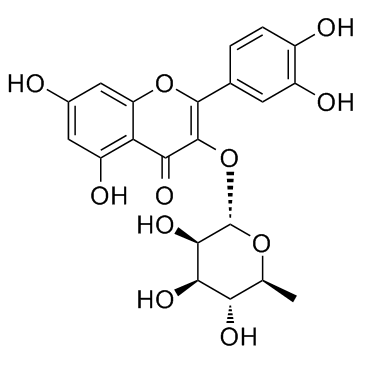
| Name | quercitrin |
|---|---|
| Synonyms |
quercetin 3-O-α-L-rhamnopyranoside
quercetin-3-L-rhamnoside 3-O-α-rhamnosylquercetine Quercetin 3-O-L-rhamnoside Quercitroside QUERCETIN 3-L-RHAMNOSIDE EINECS 208-322-5 Thujin 3,3',4',5,7-Pentahydroxyflavone 3-(6-deoxy-a-L-mannopyranoside) 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-on 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one Quercetin 3-O-α-L-rhamnoside 2-(3,4-Dihydroxyphényl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}-4H-chromén-4-one Quercetin-3-O-α-L-rhamnopyranoside 3-[(6-Deoxy-α-L-mannopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-benzopyran-4-one Quercimelin 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl-6-deoxy-α-L-mannopyranoside Quercetin-3-rhamnoside Quercetrin FLAVIN Quercetin-3-O-rhamnoside 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 6-deoxy-α-L-mannopyranoside usafcf-2 quercetin 3-O-rhamnoside MFCD00016932 3-[(6-Deoxy-a-L-mannopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one Quercitrin quercetin 3-rhamnoside |
| Description | Quercitrin is a natural compound found in Tartary buckwheat with a potential anti-inflammation effect that is used to treat heart and vascular conditions.IC50 value:Target:In vitro: There were significant increases in caspase-3 activity, loss of MMP, and increases in the apoptotic cell population in response to quercitrin in DLD-1 colon cancer cells in a time- and dose-dependent manner. [1] In vivo: ICR mice received CCl4 intraperitoneally with or without quercitrin co-administration for 4 weeks. Data showed that quercitrin significantly suppressed the elevation of reactive oxygen species (ROS) production and malondialdehyde (MDA) content, reduced tissue plasminogen activator (t-PA) activity, enhanced the antioxidant enzyme activities and abrogated cytochrome P450 2E1 (CYP2E1) induction in mouse brains. [2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 814.0±65.0 °C at 760 mmHg |
| Melting Point | 174-183ºC |
| Molecular Formula | C21H20O11 |
| Molecular Weight | 448.377 |
| Flash Point | 288.3±27.8 °C |
| Exact Mass | 448.100555 |
| PSA | 190.28000 |
| LogP | 2.17 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.776 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UZ5950000 |
|
~% 
522-12-3 |
| Literature: Isobe, Takahiko; Kanazawa, Keiji; Fujimura, Makoto; Noda, Yukinao Bulletin of the Chemical Society of Japan, 1981 , vol. 54, # 10 p. 3239 |
| Precursor 1 | |
|---|---|
| DownStream 4 | |