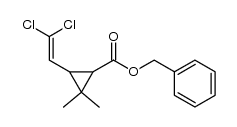
氯菊酯

氯菊酯结构式

|
常用名 | 氯菊酯 | 英文名 | Permethrin |
|---|---|---|---|---|
| CAS号 | 52645-53-1 | 分子量 | 391.288 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 465.9±45.0 °C at 760 mmHg | |
| 分子式 | C21H20Cl2O3 | 熔点 | 34-35°C | |
| MSDS | 中文版 美版 | 闪点 | 159.4±27.7 °C | |
| 符号 |



GHS02, GHS07, GHS09 |
信号词 | Danger |
氯菊酯用途Permethrin (NRDC-143) 是杀昆虫化合物,神经毒剂。 |
| 中文名 | 氯菊酯 |
|---|---|
| 英文名 | permethrin |
| 中文别名 | (3-苯氧苄基)甲基顺式,反式(±)-3-(2,2-二氯乙烯基)-2,2-二甲基环丙烷羧酸酯 | 氯菊脂(异构体) | 氯菊脂 | 除虫精 | 二氯苯醚菊酯 | 苄氯菊酯 |
| 英文别名 | 更多 |
| 描述 | Permethrin (NRDC-143) 是杀昆虫化合物,神经毒剂。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 465.9±45.0 °C at 760 mmHg |
| 熔点 | 34-35°C |
| 分子式 | C21H20Cl2O3 |
| 分子量 | 391.288 |
| 闪点 | 159.4±27.7 °C |
| 精确质量 | 390.078949 |
| PSA | 35.53000 |
| LogP | 7.15 |
| InChIKey | RLLPVAHGXHCWKJ-UHFFFAOYSA-N |
| SMILES | CC1(C)C(C=C(Cl)Cl)C1C(=O)OCc1cccc(Oc2ccccc2)c1 |
| 外观性状 | 无色晶体(但技术产品通常作为一种淡棕色液体供应) |
| 蒸汽压 | 0.0±1.2 mmHg at 25°C |
| 折射率 | 1.616 |
| 储存条件 | 库房通风低温干燥,与氧化剂分开储运 |
| 稳定性 | 对兔皮肤无刺激作用,对眼睛有轻微刺激作用。大鼠体内蓄积性小,动物试验未发现致畸、致癌、致突变作用。 |
| 水溶解性 | insoluble |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:3 4.可旋转化学键数量:7 5.互变异构体数量:无 6.拓扑分子极性表面积35.5 7.重原子数量:26 8.表面电荷:0 9.复杂度:521 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:2 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:纯品为固体,原药为棕黄色黏稠液体或半固体。 2. 密度(g/mL,20℃):1.21 30℃时密度为1.202 3. 相对蒸汽密度(g/mL,空气=1):不确定 4. 熔点(ºC):34~35 5. 沸点(ºC,0.67kpa或5 mmHg):200℃/1.33Pa、220℃/39.99Pa 6. 折射率:1.5690 7. 闪点(ºC):>200 8. 比旋光度(ºC):不确定 9. 自燃点或引燃温度(ºC)不确定 10. 蒸气压(kPa,25ºC):4.53×10-5 11. 饱和蒸气压(kPa,60ºC):不确定 12. 燃烧热(KJ/mol):不确定 13. 临界温度(ºC):不确定 14. 临界压力(KPa):不确定 15. 油水(辛醇/水)分配系数的对数值:不确定 16. 爆炸上限(%,V/V):不确定 17. 爆炸下限(%,V/V):不确定 18. 溶解性:不确定 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
氯菊酯毒理学数据: 原药对大鼠急性经日LD50为1200 - 2000mg /kg,急性吸入LC50 >23.Sg/m' (4h),大鼠和兔急性经皮LD50> 2000mg/kg。以1500mg /kg剂量喂养大鼠6个月无影响。虹蝉鱼、蓝鳃鱼LC50 为0 . 0032mg几(96h)>鹤鹑急性经日LD50为13500mg/kg>蜜蜂接触LD50为0.1μg/只、经日0.2μg/只。 |
| 符号 |



GHS02, GHS07, GHS09 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H225-H302-H312-H319-H332-H411 |
| 警示性声明 | P210-P273-P280-P305 + P351 + P338 |
| 个人防护装备 | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| 危害码 (欧洲) | Xn: Harmful;N: Dangerous for the environment;T: Toxic;F: Flammable; |
| 风险声明 (欧洲) | R20/22 |
| 安全声明 (欧洲) | S13-S24-S36/37/39-S60-S61-S45-S36/37-S16-S7-S26 |
| 危险品运输编码 | UN1230 3/PG 2 |
| RTECS号 | GZ1255000 |
| 海关编码 | 2916209022 |
|
~76% 
氯菊酯 52645-53-1 |
| 文献:Sagami Chemical Research Center Patent: US4214097 A1, 1980 ; |
|
~93% 
氯菊酯 52645-53-1 |
| 文献:Bayer Aktiengesellschaft Patent: US4225704 A1, 1980 ; |
|
~95% 
氯菊酯 52645-53-1 |
| 文献:Nakatsuji, Hidefumi; Morita, Jun-Ichi; Misaki, Tomonori; Tanabe, Yoo Advanced Synthesis and Catalysis, 2006 , vol. 348, # 15 p. 2057 - 2062 |
|
~% 
氯菊酯 52645-53-1 |
| 文献:Kuraray Co., Ltd. Patent: US4113968 A1, 1978 ; |
|
~% 
氯菊酯 52645-53-1 |
| 文献:Sumitomo Chemical Company, Limited Patent: US6441220 B1, 2002 ; |
|
详细
|
| 文献:Sagami Chemical ResearchCenter Patent: US4500733 A1, 1985 ; |
|
~% 
氯菊酯 52645-53-1 |
| 文献:Sumitomo Chemical Company, Limited Patent: US6531626 B1, 2003 ; |
|
~% 
氯菊酯 52645-53-1 |
| 文献:Sumitomo Chemical Company, Limited Patent: US6531626 B1, 2003 ; |
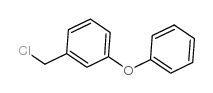
| 氯菊酯上游产品 9 | |
|---|---|
| 氯菊酯下游产品 2 | |
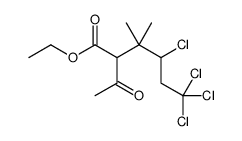
二氯乙醛与异丁烯作用得(上)(II)两个二氯烯醉同分异构体。反应以二氯化铝为催化剂,在低温下进行。在氯化氢存在下,乙睛与无水乙醉作用生成业氦基乙醚盐酸盐,进一步与无水乙醉反应生成原乙酸三乙酯(Ⅲ)。在催化剂对甲苯磺酸存在下,加热至114~118ºC,反应3h,使(上)转化为(II)。将转位产物与原乙酸二乙酷作用,催化剂为异丁酸,反应温度从110℃上升至150 ºC,大约12h,在150~155℃反应7~8h,于0.08MPa压力下蒸出前馏分,得3,3一二甲基-4,6,6一二氯已烯一[5〕一酸乙酷(V),并伴随(11)。将(11)在氯化氢一乙醉洛液中进行开环反应又得(V)。(V)在乙醉钠存在下,于40 ~ 45℃反应Sh,环合生成2,2一二甲基W2, 2一二氯乙烯基)一环丙烷拔酸乙酷C VII },经精馏得纯度较高的二氯菊酸乙酷。在碱液存在下,将(1'1l)回流反应3h,皂化得相应钠盐(VIII)。 (VIII)与氯化间苯氧基节基二乙胺反应,制得氯菊酷(IX)。反应过程如下:
| 海关编码 | 2916209022 |
|---|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals ... |
| Anomethrin N |
| 3-(Phenoxyphenyl)methyl (±)-cis,trans-3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| kudos |
| Ambushfog |
| Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester |
| Peregin |
| Stomozan |
| Perigen |
| Kestrel |
| Pounce |
| Peregin W |
| SBP 15131TEC |
| 3-Phenoxybenzyl-3-(2,2-dichlorethenyl)-2,2-dimethylcyclopropancarboxylat |
| Chinetrin |
| Outflank |
| L3TJ A1 A1 BVO1R COR&& C1UYGG |
| Permanone 80 |
| ICI-PP 557 |
| Picket G |
| 1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Exmin |
| EXPAR |
| 3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Eksmin |
| Kafil |
| Stomoxin |
| 3-phenoxybenzyl (1RS,3RS |
| Indothrin |
| Cosair |
| BW-21-Z |
| Quamlin |
| Ambush |
| Kavil |
| Ridect Pour-On |
| Talcord |
| (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| Outflank-stockade |
| 3-Dichloroacetyl-4-hydroxy-1-methyl-2-oxo-1,2-dihydroquinoline |
| Insorbcid MP |
| MFCD00041809 |
| Efmethrin |
| (3-Phenoxyphenyl)methyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Lyclear |
| Ecsumin |
| Perigen W |
| Diffusil H |
| m-Phenoxybenzyl (±)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| [3-(phenyloxy)phenyl]methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| Permethrin |
| (±)-3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| (3-Phenoxyphenyl)methyl 3-(2,2-dichlorethenyl)-2,2-dimethylcyclopropanecarboxylate |
| Imperator |
| Coopex |
| m-Phenoxybenzyl (±)-cis,trans-3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Stomoxi |
| Permethrin (cis/trans) |
| Acticin |
| Picket |
| Permasect-25EC |
| 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (3-phenoxyphenyl)methyl ester |
| Bematin 987 |
| Permasect |
| SBP-1513TEC |
| Pynosect |
| Exsmin |
| 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid, (3-phenoxyphenyl)methyl ester |
| MP79 |
| Permethrin (isomers) |
| 3-phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2- dimethylcyclopropanecarboxylate |
| (3-Phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate |
| LE 79-519 |
| (3-phenoxyphenyl)methyl (1Ξ,3Ξ)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate |
| Antiborer 3768 |
| Kaleait |
| 3-Phenoxybenzyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| EINECS 258-067-9 |
| Stomoxin P |
| Dragnet FT |
| Ectiban |
| Mitin BC |
| (3-Phenoxyphenyl)methyl (±)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| Ipitox |
| Pramex |
| 3-Phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Corsair |
| NIX |
| Dragnet |
| 3-Phenoxybenzyl 2,2-dimethyl-3-(2,2-dichlorovinyl)cyclopropanecarboxylate |
| Cooper |
| Perthrine |
| SBP-3246 |

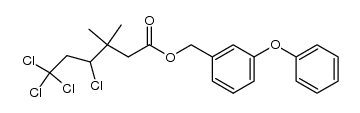
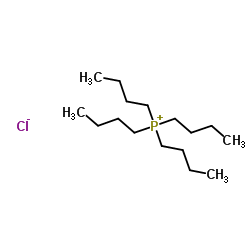

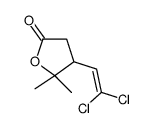
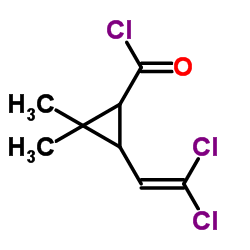


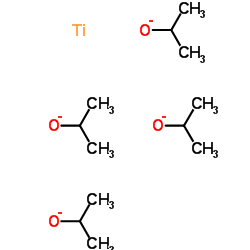
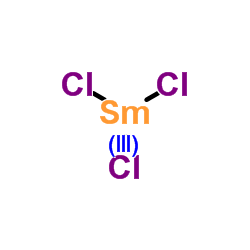
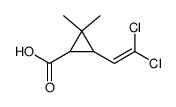 CAS号55701-05-8
CAS号55701-05-8
