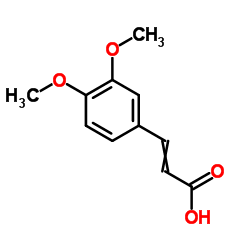
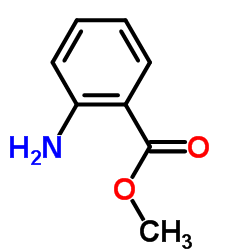
Tranilast
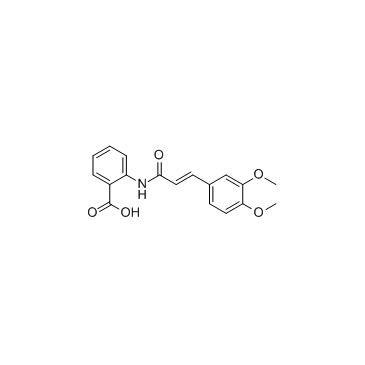
Tranilast structure
|
Common Name | Tranilast | ||
|---|---|---|---|---|
| CAS Number | 53902-12-8 | Molecular Weight | 327.331 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 585.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C18H17NO5 | Melting Point | 166-168ºC | |
| MSDS | Chinese USA | Flash Point | 307.9±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of TranilastTranilast is an antiallergic agent.Target: Angiotensin ReceptorTranilast has been approved in Japan and South Korea, since 1982, for the treatment of bronchial asthma, with indications for keloids and hypertrophic scar added in 1993. Tranilast is also used to treat asthma, autoimmune diseases, atopic and fibrotic pathologies, and can also inhibit angiogenesis. The antiproliferative properties of tranilast were found that tranilast elicited an inhibitory effect on fibroblast proliferation in vitro and also suppressed collagen production both in vitro and in vivo . Tranilast also reduced the release of chemical mediators from mast cells and suppressed hypersensitivity reactions. [1]Three-week-old C57Bl/10 and mdx mice received tranilast (~300 mg/kg) in their food for 9 weeks, after which fibrosis was assessed through histological analyses, and functional properties of tibialis anterior muscles were assessed in situ and diaphragm muscle strips in vitro. Tranilast administration did not significantly alter the mass of any muscles in control or mdx mice, but it decreased fibrosis in the severely affected diaphragm muscle by 31% compared with untreated mdx mice (P< 0.05) [2]. |
| Name | tranilast |
|---|---|
| Synonym | More Synonyms |
| Description | Tranilast is an antiallergic agent.Target: Angiotensin ReceptorTranilast has been approved in Japan and South Korea, since 1982, for the treatment of bronchial asthma, with indications for keloids and hypertrophic scar added in 1993. Tranilast is also used to treat asthma, autoimmune diseases, atopic and fibrotic pathologies, and can also inhibit angiogenesis. The antiproliferative properties of tranilast were found that tranilast elicited an inhibitory effect on fibroblast proliferation in vitro and also suppressed collagen production both in vitro and in vivo . Tranilast also reduced the release of chemical mediators from mast cells and suppressed hypersensitivity reactions. [1]Three-week-old C57Bl/10 and mdx mice received tranilast (~300 mg/kg) in their food for 9 weeks, after which fibrosis was assessed through histological analyses, and functional properties of tibialis anterior muscles were assessed in situ and diaphragm muscle strips in vitro. Tranilast administration did not significantly alter the mass of any muscles in control or mdx mice, but it decreased fibrosis in the severely affected diaphragm muscle by 31% compared with untreated mdx mice (P< 0.05) [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 585.5±50.0 °C at 760 mmHg |
| Melting Point | 166-168ºC |
| Molecular Formula | C18H17NO5 |
| Molecular Weight | 327.331 |
| Flash Point | 307.9±30.1 °C |
| Exact Mass | 327.110687 |
| PSA | 84.86000 |
| LogP | 4.36 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.648 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 18 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DG8731000 |
|
~% 
Tranilast CAS#:53902-12-8 |
| Literature: US3940422 A1, ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Osmosensation in TRPV2 dominant negative expressing skeletal muscle fibres.
J. Physiol. 593 , 3849-63, (2015) Increased plasma osmolarity induces intracellular water depletion and cell shrinkage (CS) followed by activation of a regulatory volume increase (RVI). In skeletal muscle, the hyperosmotic shock-induc... |
|
|
Collagen density regulates xenobiotic and hypoxic response of mammary epithelial cells.
Environ. Toxicol. Pharmacol. 39(1) , 114-24, (2015) Breast density, where collagen I is the dominant component, is a significant breast cancer risk factor. Cell surface integrins interact with collagen, activate focal adhesion kinase (FAK), and downstr... |
|
|
Inhibitory Effect of Tranilast on Transforming Growth Factor-Beta-Induced Protein in Granular Corneal Dystrophy Type 2 Corneal Fibroblasts.
Cornea 34 , 950-8, (2015) To investigate the effects of tranilast, an inhibitor of chemical mediators and fibroblast proliferation, on the expression of transforming growth factor-beta (TGF-β)-induced protein (TGFBIp) in wild-... |
| 2-({(2E)-3-[3,4-bis(methyloxy)phenyl]prop-2-enoyl}amino)benzoic acid |
| 2-{[(2E)-3-(3,4-Dimethoxyphenyl)prop-2-enoyl]amino}benzoic acid |
| (E)-2-(3-(3,4-dimethoxyphenyl)acrylamido)benzoic acid |
| benzoic acid, 2-[[(2E)-3-(3,4-dimethoxyphenyl)-1-oxo-2-propenyl]amino]- |
| 2-{[(2E)-3-(3,4-Dimethoxyphenyl)-2-propenoyl]amino}benzoic acid |
| N-(3,4-Dimethoxycinnamoyl)anthranilic Acid |
| 2-[[3-(3,4-Dimethoxyphenyl)-1-oxo-2-propenyl]amino]benzoic Acid |
| Tranilast |
| rizaben |
| Rizaben,MK 341,Tranpro |
| MFCD00864787 |
| MK 341 |
| Tranpro |
| Benzoic acid, 2-[[(2E)-3-(3,4-dimethoxyphenyl)-1-oxo-2-propen-1-yl]amino]- |