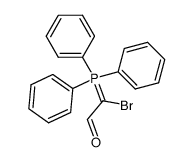
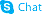α-Bromocinnamaldehyde
α-Bromocinnamaldehyde structure
|
Common Name | α-Bromocinnamaldehyde | ||
|---|---|---|---|---|
| CAS Number | 5443-49-2 | Molecular Weight | 211.055 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 304.4±30.0 °C at 760 mmHg | |
| Molecular Formula | C9H7BrO | Melting Point | 66-68 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 110.2±11.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2-Bromocinnamaldehyde |
|---|---|
| Synonym | More Synonyms |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 304.4±30.0 °C at 760 mmHg |
| Melting Point | 66-68 °C(lit.) |
| Molecular Formula | C9H7BrO |
| Molecular Weight | 211.055 |
| Flash Point | 110.2±11.9 °C |
| Exact Mass | 209.968018 |
| PSA | 17.07000 |
| LogP | 2.44 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.627 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36/37/39-S22 |
| RIDADR | UN 3335 |
| WGK Germany | 3 |
| RTECS | GD6480000 |
| HS Code | 2913000090 |
|
~83% 
α-Bromocinnamal... CAS#:5443-49-2 |
| Literature: Saraf, S. D. Journal fuer Praktische Chemie (Leipzig), 1981 , vol. 323, # 4 p. 673 - 676 |
|
~92% 
α-Bromocinnamal... CAS#:5443-49-2 |
| Literature: Yao, Changsheng; Wang, Donglin; Lu, Jun; Li, Tuanjie; Jiao, Weihui; Yu, Chenxia Chemistry - A European Journal, 2012 , vol. 18, # 7 p. 1914 - 1917 |
|
~42% 
α-Bromocinnamal... CAS#:5443-49-2 |
| Literature: Migalina, Yu. V.; Galla-Bobik, S. V.; Lendel, V. G.; Staninets, V. I. J. Gen. Chem. USSR (Engl. Transl.), 1982 , vol. 52, # 7 p. 1563 - 1566,1380 - 1382 |
|
~% 
α-Bromocinnamal... CAS#:5443-49-2 |
| Literature: Chemische Berichte, , vol. 95, p. 3003 - 3007 |
|
~% 
α-Bromocinnamal... CAS#:5443-49-2 |
| Literature: Chemische Berichte, , vol. 17, p. 1815 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2913000090 |
|---|---|
| Summary | HS: 2913000090 halogenated, sulphonated, nitrated or nitrosated derivatives of products of heading 2912 Educational tariff:17.0% Tax rebate rate:9.0% Regulatory conditions:none Most favored nation tariff:5.5% General tariff:30.0% |
|
Masuda borylation-Suzuki coupling (MBSC) sequence of vinylhalides and its application in a one-pot synthesis of 3,4-biarylpyrazoles.
Org. Biomol. Chem. 11(36) , 6113-8, (2013) The Masuda borylation-Suzuki coupling (MBSC) sequence was successfully extended to the challenging coupling of vinylhalides with various (hetero)arylhalides using sterically hindered phosphane ligands... |
|
|
[Alpha-bromocinnamaldehyde, its mutagenicity and contents in commercial products].
Eisei Shikenjo Hokoku. (107) , 21-5, (1989) The amount of alpha-bromocinnamaldehyde (BCA), an anti-mildew agent, in some commercial products, was examined by high performance liquid chromatography (HPLC) using the following conditions: column, ... |
|
|
Guanidinium ylide mediated aziridination: identification of a spiro imidazolidine-oxazolidine intermediate.
J. Org. Chem. 71(17) , 6600-3, (2006) We successfully isolated a spiro imidazolidine-oxazolidine intermediate in the reaction of guanidinium ylide mediated aziridination using alpha-bromocinnamaldehyde. X-ray crystallographic analysis una... |
| A-BROMOCINNAMIC ACID |
| b36 |
| b37 |
| 2-bromo-3-phenylpropenal |
| α-Bromocinnamic aldehyde |
| (2Z)-2-bromo-3-phenylprop-2-enal |
| 2-Propenal, 2-bromo-3-phenyl-, (Z)- |
| EINECS 226-637-6 |
| Cinnamaldehyde, α-bromo- |
| BROMOCINNAMAL |
| 2-Propenal, 2-bromo-3-phenyl-, (2Z)- |
| Alphabrocine |
| a-Bromocinnamaldehyde |
| 2-bromo-3-phenylacrylaldehyde |
| MFCD00006965 |
| 2-bromo-3-phenylacrolein |
| (2Z)-2-Bromo-3-phenylacrylaldehyde |
| α-Bromocinnamaldehyde |

![2-[(7-amino-[1,2,4]triazolo[1,5-c]pyrimidin-5-yl)sulfanyl]-N-[(E)-[(Z)-2-bromo-3-phenylprop-2-enylidene]amino]acetamide structure](https://www.chemsrc.com/caspic/395/143212-87-7.png) CAS#:143212-87-7
CAS#:143212-87-7 CAS#:103-26-4
CAS#:103-26-4 CAS#:1219618-77-5
CAS#:1219618-77-5 CAS#:1219618-68-4
CAS#:1219618-68-4 CAS#:1219618-74-2
CAS#:1219618-74-2 CAS#:1866-31-5
CAS#:1866-31-5 CAS#:7779-17-1
CAS#:7779-17-1 CAS#:103-41-3
CAS#:103-41-3 CAS#:6142-95-6
CAS#:6142-95-6