| Description |
Sunitinib (SU 11248) is a multi-targeted receptor tyrosine kinase inhibitor with IC50s of 80 nM and 2 nM for VEGFR2 and PDGFRβ, respectively.
|
| Related Catalog |
|
| Target |
VEGFR2:80 nM (IC50)
PDGFRβ:2 nM (IC50)
|
| In Vitro |
Sunitinib Malate is also a good inhibitor of KIT and FLT-3[1]. In biochemical assays, Sunitinib (SU11248) exhibits competitive inhibition (with regard to ATP) against Flk-1 and PDGFRβ with Ki values of 9 nM and 8 nM, respectively. Sunitinib is also a competitive, albeit less potent, inhibitor of FGFR1 tyrosine kinase activity, with a Ki value of 0.83 μM. In addition to these three structurally related split kinase domain RTKs, the activity of Sunitinib has also been evaluated against a broad panel of additional tyrosine and serine/threonine kinases. In these biochemical assays, the IC50 values for Sunitinib are generally at least 10-fold higher than those for Flk-1 and PDGFR (e.g., IC50values of: >10 μM for EGFR and Cdk2; 4 μM for Met; 2.4 μM for IGFR-1; 0.8 μM for Abl; and 0.6 μM for Src)[2]. In RS4;11 cells (FLT3-WT), treatment with Sunitinib (SU11248) inhibits FLT3-WT phosphorylation in a dose-dependent manner with IC50 of approximately 250 nM. In MV4;11 cells that express FLT3-ITD, Sunitinib inhibits FLT3-ITD phosphorylation in a dose-dependent manner with an IC50 of 50 nM following a 2-hour treatment[3].
|
| In Vivo |
Sunitinib Malate has very good oral bioavailability, is highly efficacious in a number of preclinical tumor models, and is well tolerated at efficacious doses[1]. Sunitinib (80 mg/kg/day) inhibits the growth of established SF763T and Colo205 tumor xenografts in athymic mice. Sunitinib (SU11248) treatment effectively inhibits the growth of established tumor xenografts[2]. Sunitinib malate is an inhibitor of VEGFR, PDGFR, FGFR, and is used in the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumors. Sunitinib malate-treated rats display much lower levels of tumor growth than untreated rats, and their tumors have much smaller necrotic areas and lower vascular density[4].
|
| Cell Assay |
RS4;11 and MV4;11 cell lines are starved overnight in medium containing 0.1% FBS prior to addition of Sunitinib (1 nM, 5 nM, 10 nM, 25 nM, 75 nM, 100 nM, 250 nM, 500 nM) and FL (50 ng/mL; FLT3-WT cells only). Proliferation is measured after 48 hours of culture using the Alamar Blue assay in triplicate for each condition, as described by the manufacturer. Trypan blue cell viability assays are performed in parallel and yielded similar results[3].
|
| Animal Admin |
Mice[2] Female nu/nu mice (8-12 weeks old, 25 grams) are used. Briefly, 3-5×106 tumor cells are implanted s.c. into the hind flank region of mice on day 0. Daily treatment of tumor-bearing mice with oral administration of Sunitinib as a carboxymethyl cellulose suspension or as a citrate buffered (pH 3.5) solution is initiated once the tumors reached the indicated average size. Tumor growth is evaluated based on twice-weekly measurement of tumor volume. Typically, studies are terminated when tumors in vehicle-treated animals reach an average size of 1000 mm3 or when the tumors are judged to adversely effect the well being of the animals. Rats[4] Adult male Wistar rats (325-349 g) are used. To validate the ability of the time-lapse imaging method to evaluate the anti-angiogenic effects for a given drug treatment, two drug studies are conducted. In the first study, mesenteric windows are harvested from adult male Wistar rats and cultured for 3 days according to the two experimental groups: 1) 10% serum (n=8 tissues from 4 rats), and 2) 10% serum+Sunitinib (5 μM; n=8 tissues from 4 rats).
|
| References |
[1]. Sun L, et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor r [2]. Mendel DB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Can [3]. O'Farrell AM, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003 May 1;101(9):3597-605. [4]. Azimi MS, et al. An ex vivo model for anti-angiogenic drug testing on intact microvascular networks. PLoS One. 2015 Mar 5;10(3):e0119227.
|
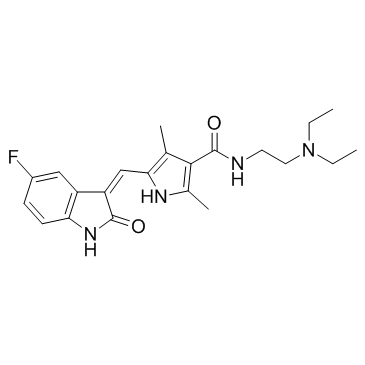
 CAS#:100-36-7
CAS#:100-36-7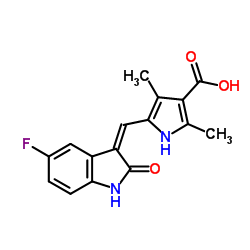 CAS#:356068-93-4
CAS#:356068-93-4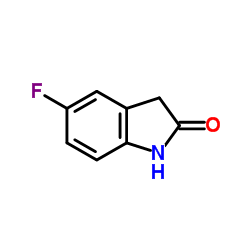 CAS#:56341-41-4
CAS#:56341-41-4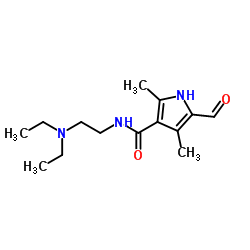 CAS#:356068-86-5
CAS#:356068-86-5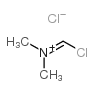 CAS#:3724-43-4
CAS#:3724-43-4![N-[2-(DiethylaMino)ethyl]-2,4-diMethyl-1H-pyrrole-3-CarboxaMide Structure](https://image.chemsrc.com/caspic/431/590424-05-8.png) CAS#:590424-05-8
CAS#:590424-05-8![(Z)-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl 5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylate Structure](https://image.chemsrc.com/caspic/211/452105-55-4.png) CAS#:452105-55-4
CAS#:452105-55-4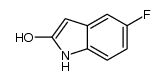 CAS#:1032268-12-4
CAS#:1032268-12-4 CAS#:1338703-87-9
CAS#:1338703-87-9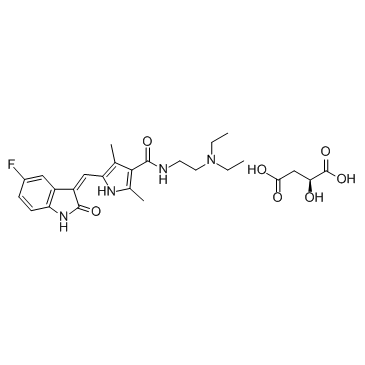 CAS#:341031-54-7
CAS#:341031-54-7
