Quinidine

Quinidine structure
|
Common Name | Quinidine | ||
|---|---|---|---|---|
| CAS Number | 56-54-2 | Molecular Weight | 324.417 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 495.9±40.0 °C at 760 mmHg | |
| Molecular Formula | C20H24N2O2 | Melting Point | 168-172 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 253.7±27.3 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of QuinidineQuinidine is an antiarrhythmic agent for the treatment of abnormal heart rhythms and also malaria. |
| Name | quinidine |
|---|---|
| Synonym | More Synonyms |
| Description | Quinidine is an antiarrhythmic agent for the treatment of abnormal heart rhythms and also malaria. |
|---|---|
| Related Catalog | |
| Target |
Parasite[1] |
| In Vitro | Quinidine is a clinical anti-arrythmic drug which affects ionic currents in heart muscle and which has also been shown to be a potent blocker of several classes of K+ channel in a variety of cell types. Bath application of quinidine causes a dose-dependent reduction of the peak amplitude of Ik. The Kd for blockade of Ik at 0 mV is estimated to be 41 μM. Quinidine elicits a dose-dependent increase of the rate of the decay of Ik and this effect is enhanced by membrane depolarization. Quinidine also causes a 5 mV hyperpolarizing shift of the steady-state inactivation curve and increases the half-time for recovery from inactivation. Quinidine does not affect the onset of inactivation measured at -30 mV[1]. |
| In Vivo | Quinidine sulfate is rapidly absorbed, with peak plasma concentrations 60-90 min after an oral dose. Other salts (gluconate, polygalacturonate) are more slowly absorbed, with lower peak concentrations. Quinidine is approximately 70-90 % bound to plasma proteins. It undergoes hepatic oxidative metabolism to form an N-oxide, a 3-hydroxy form, an O-demethyl form and 2'-quinidinone. Over one-half of patients starting quinidine stop within the first year of therapy because of side effects. These include, commonly, diarrhea, nausea, and vomiting which are not necessarily related to high plasma concentrations[2]. Quinidine inhibits metabolism of amphetamine in rats. Quinidine pretreatment results in a significant decrease in the excretion of p-hydroxyamphetamine at 24 and 48 h to 7.2 and 24.1% of the vehicle-control levels, respectively, accompanied by a significant increase in amphetamine excretion between 24 and 48 h to 542% of the control[3]. |
| Animal Admin | Rats: Three rats are placed in individual metabolic cages with free access to food and water. After 24 h, urine is collected (0 h) and the rats receive the following treatment: (1) no treatment; (2) 80 mg quinidine/kg (po) at 1.0 mL/kg, and (III) 50% ethanol (1.0 mL/kg). Two hours later, all three rats receive a single dose of 15 mg amphetamine/kg (po) at 1.0 mL/kg. Urine is then collected at 24 and 48 h after the administration of quinidine. This procedure is repeated three times. After collection of urine, volume and pH are measured and the urine is stored until analysis[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 495.9±40.0 °C at 760 mmHg |
| Melting Point | 168-172 °C(lit.) |
| Molecular Formula | C20H24N2O2 |
| Molecular Weight | 324.417 |
| Flash Point | 253.7±27.3 °C |
| Exact Mass | 324.183777 |
| PSA | 45.59000 |
| LogP | 3.44 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.638 |
| InChIKey | LOUPRKONTZGTKE-LHHVKLHASA-N |
| SMILES | C=CC1CN2CCC1CC2C(O)c1ccnc2ccc(OC)cc12 |
| Water Solubility | 0.05 g/100 mL (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S36-S22 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | VA4725000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Organic anion transporting polypeptides and organic cation transporter 1 contribute to the cellular uptake of the flavonoid quercetin.
Naunyn Schmiedebergs Arch. Pharmacol. 387(9) , 883-91, (2014) Flavonoids such as quercetin and kaempferol mediate several health protective effects, e.g., anticancer effects. They are inhibitors of organic anion transporting polypeptides (OATP) and organic catio... |
|
|
Morphological behaviour and metabolic capacity of cryopreserved human primary hepatocytes cultivated in a perfused multiwell device.
Xenobiotica 45(1) , 29-44, (2014) 1. The quantitative prediction of the pharmacokinetic parameters of a drug from data obtained using human in vitro systems remains a significant challenge i.e. prediction of metabolic clearance in hum... |
|
|
Utility of cerebrospinal fluid drug concentration as a surrogate for unbound brain concentration in nonhuman primates.
Drug Metab. Pharmacokinet. 29(5) , 419-26, (2014) In central nervous system drug discovery, cerebrospinal fluid (CSF) drug concentration (C(CSF)) has been widely used as a surrogate for unbound brain concentrations (C(u,brain)). However, previous rod... |
| Conchinin |
| Chinidin |
| (S)-(6-Methoxy-4-quinolinyl)[(2R,4S,5R)-5-vinyl-1-azabicyclo[2.2.2]oct-2-yl]methanol |
| Cin-quin |
| (8R,9S)-6'-Methoxycinchonan-9-ol |
| Coccinine |
| (9S)-6'-Methoxycinchonan-9-ol |
| Quinidex |
| (S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]oct-2-yl](6-methoxyquinolin-4-yl)methanol |
| Quinidine,(S)-(6-Methoxyquinolin-4-yl)((2R,4S,8R)-8-vinylquinuclidin-2-yl)methanolhydrochloride |
| Cinchonan-9-ol, 6'-methoxy-, (9S)- |
| Quinidine [BAN] |
| Raclopride |
| cinchonan-9-ol, 6'-methoxy-, (3a,9S)- |
| QUINDINE |
| (9S)-6-Methoxy-a-(5-vinyl-2-quinuclidinyl)-4-quinolinemethanol |
| TCMDC-131239 |
| (S)-(6-Methoxy-4-chinolinyl)[(2R,4S,5R)-5-vinyl-1-azabicyclo[2.2.2]oct-2-yl]methanol |
| Kinidin |
| MFCD00135581 |
| (S)-[(2R,4S,5R)-5-éthènyl-1-azabicyclo[2.2.2]oct-2-yl](6-méthoxy-4-quinoléinyl)méthanol |
| QUNIDINE |
| PITAYINE |
| Quinidine |
| (3a,9S)-6'-methoxycinchonan-9-ol |
| Pitayin |
| EINECS 200-279-0 |
| (9S)-6-Methoxy-α-(5-vinyl-2-quinuclidinyl)-4-quinolinemethanol |
 CAS#:56652-53-0
CAS#:56652-53-0![(4S)-[3-(6-methoxyquinolin-4-yl)-(S,S)-oxiranylmethyl]-(3R)-vinylpiperidine-1-carboxylic acid benzyl ester Structure](https://image.chemsrc.com/caspic/256/657408-10-1.png) CAS#:657408-10-1
CAS#:657408-10-1 CAS#:4363-94-4
CAS#:4363-94-4 CAS#:865853-19-6
CAS#:865853-19-6 CAS#:865853-23-2
CAS#:865853-23-2 CAS#:865853-22-1
CAS#:865853-22-1 CAS#:865853-21-0
CAS#:865853-21-0 CAS#:865853-20-9
CAS#:865853-20-9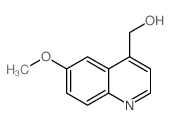 CAS#:92288-15-8
CAS#:92288-15-8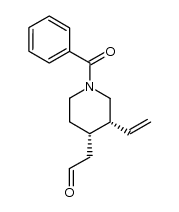 CAS#:35189-37-8
CAS#:35189-37-8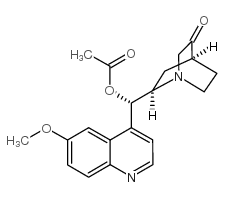 CAS#:60723-43-5
CAS#:60723-43-5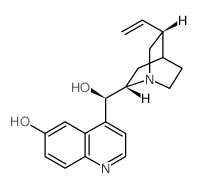 CAS#:524-63-0
CAS#:524-63-0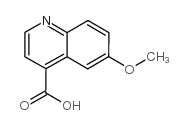 CAS#:86-68-0
CAS#:86-68-0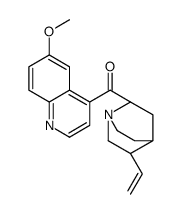 CAS#:84-31-1
CAS#:84-31-1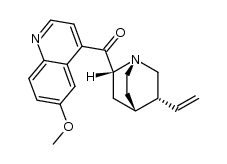 CAS#:14528-53-1
CAS#:14528-53-1 CAS#:84-55-9
CAS#:84-55-9 CAS#:77481-82-4
CAS#:77481-82-4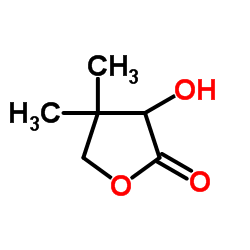 CAS#:599-04-2
CAS#:599-04-2 CAS#:135754-90-4
CAS#:135754-90-4
