Diacetyl monoxime
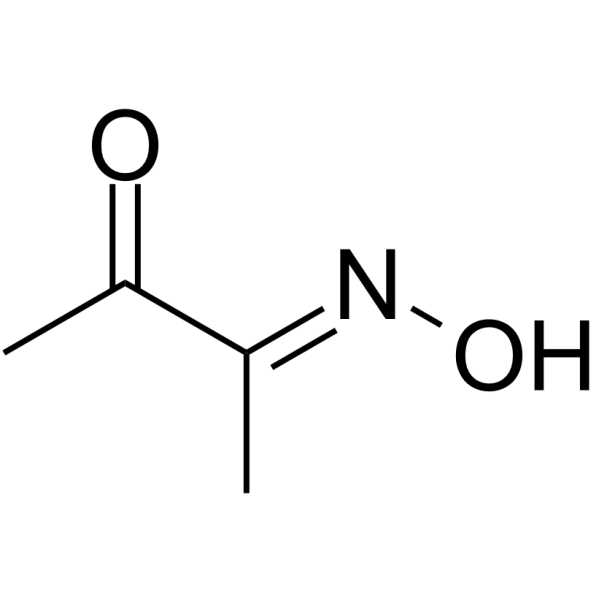
Diacetyl monoxime structure
|
Common Name | Diacetyl monoxime | ||
|---|---|---|---|---|
| CAS Number | 57-71-6 | Molecular Weight | 101.104 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 185.5±9.0 °C at 760 mmHg | |
| Molecular Formula | C4H7NO2 | Melting Point | 75-78 °C(lit.) | |
| MSDS | USA | Flash Point | 66.0±18.7 °C | |
Use of Diacetyl monoximeBiacetyl monoxime (Diacetyl monoxime), a myosin ATPase inhibitor, is a skeletal and cardiac muscle contraction inhibitor. Biacetyl monoxime induces sarcoplasmic reticulum Ca2+ release[1][2]. |
| Name | Diacetylmonoxime |
|---|---|
| Synonym | More Synonyms |
| Description | Biacetyl monoxime (Diacetyl monoxime), a myosin ATPase inhibitor, is a skeletal and cardiac muscle contraction inhibitor. Biacetyl monoxime induces sarcoplasmic reticulum Ca2+ release[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 185.5±9.0 °C at 760 mmHg |
| Melting Point | 75-78 °C(lit.) |
| Molecular Formula | C4H7NO2 |
| Molecular Weight | 101.104 |
| Flash Point | 66.0±18.7 °C |
| Exact Mass | 101.047676 |
| PSA | 49.66000 |
| LogP | -0.47 |
| Vapour Pressure | 0.3±0.7 mmHg at 25°C |
| Index of Refraction | 1.452 |
| InChIKey | FSEUPUDHEBLWJY-UHFFFAOYSA-N |
| SMILES | CC(=O)C(C)=NO |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | 5 g/100 mL (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S22-S24/25-S36/37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | EK3150000 |
| HS Code | 29280090 |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Carbamylation of immunoglobulin abrogates activation of the classical complement pathway.
Eur. J. Immunol. 44(11) , 3403-12, (2014) Post-translational modifications of proteins significantly affect their structure and function. The carbamylation of positively charged lysine residues to form neutral homoitrulline occurs primarily u... |
|
|
Elastic proteins in the flight muscle of Manduca sexta.
Arch. Biochem. Biophys. 568 , 16-27, (2015) The flight muscles (DLM1) of the Hawkmoth, Manduca sexta are synchronous, requiring a neural spike for each contraction. Stress/strain curves of skinned DLM1 showed hysteresis indicating the presence ... |
|
|
Metrics and clinical relevance of percutaneous penetration and lateral spreading.
Skin Pharmacol. Physiol. 28(2) , 57-64, (2015) Percutaneous penetration of urea in vivo in man has been documented. If urea can penetrate the skin, it may also move laterally. Lateral spreading of topical substances leads to unpredictable penetrat... |
| 2,3-Butanedione, monooxime, (2E)- |
| (3E)-3-(Hydroxyimino)-2-butanone |
| EINECS 200-348-5 |
| 2,3-BUTANEDIONE-2-OXIME |
| Diacetyl monoxime |
| (2E)-butane-2,3-dione oxime |
| (3E)-3-(Hydroxyimino)butan-2-one |
| MFCD00002116 |
| 2,3-Butanedione monoxime |
 CAS#:78-93-3
CAS#:78-93-3 CAS#:110-46-3
CAS#:110-46-3 CAS#:328918-80-5
CAS#:328918-80-5![N,N-bis[(E)-3-nitrosobut-2-en-2-yl]ethane-1,2-diamine structure](https://image.chemsrc.com/caspic/078/36658-91-0.png) CAS#:36658-91-0
CAS#:36658-91-0 CAS#:7273-08-7
CAS#:7273-08-7 CAS#:41933-67-9
CAS#:41933-67-9 CAS#:6116-75-2
CAS#:6116-75-2
