Methylergometrine maleate
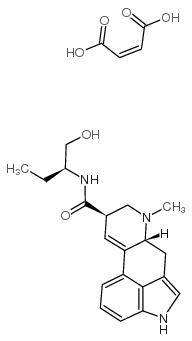
Methylergometrine maleate structure
|
Common Name | Methylergometrine maleate | ||
|---|---|---|---|---|
| CAS Number | 57432-61-8 | Molecular Weight | 455.50400 | |
| Density | 1.2744 (rough estimate) | Boiling Point | 638.4ºC at 760mmHg | |
| Molecular Formula | C24H29N3O6 | Melting Point | 172ºC (dec) | |
| MSDS | N/A | Flash Point | N/A | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
| Purity | Quantity | Budget | Inquiry |
|---|
Use of Methylergometrine maleateMethylergometrine maleate (Methylergonovine maleate) is an ergot alkaloid and an active metabolite of Methysergide with vasoconstrictive and uterotonic activity. Methylergometrine maleate is a potent, selective and orally active 5-HT receptors antagonist with a pA2 value of 9.6. Methylergometrine maleate has antimigraine and dopaminergic activity. Methylergometrine maleate can used for the prevention and control of postpartum hemorrhage[1][2][3]. |
| Name | Methyl Ergonovine Maleate Salt |
|---|---|
| Synonym | More Synonyms |
| Description | Methylergometrine maleate (Methylergonovine maleate) is an ergot alkaloid and an active metabolite of Methysergide with vasoconstrictive and uterotonic activity. Methylergometrine maleate is a potent, selective and orally active 5-HT receptors antagonist with a pA2 value of 9.6. Methylergometrine maleate has antimigraine and dopaminergic activity. Methylergometrine maleate can used for the prevention and control of postpartum hemorrhage[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
serotonin:9.6 nM (pA2) |
| In Vitro | Both Methysergide and Methylergometrine displace the concentration-response curve of 5-HT to the right and depressed the maximum effect ; thus, both agents behaved as non-competitive antagonists of 5-HT. Methylergometrine is approximately 40-times more potent than Methysergide as an antagonist of the 5-HT-induced responses, since the calculated apparent pA2 values are 9.6 and 8.0, respectively[3]. |
| In Vivo | Methylergometrine is more potent than Methysergide as a vasoconstrictor on bovine middle cerebral artery and human coronary arteries, an effect on 5-HT1B receptors. Subacute oral administration would result in metabolism of Methysergide to Methylergometrine[2]. |
| References |
[2]. Koehler PJ, et al. History of methysergide in migraine. Cephalalgia. 2008 Nov;28(11):1126-35. |
| Density | 1.2744 (rough estimate) |
|---|---|
| Boiling Point | 638.4ºC at 760mmHg |
| Melting Point | 172ºC (dec) |
| Molecular Formula | C24H29N3O6 |
| Molecular Weight | 455.50400 |
| Exact Mass | 455.20600 |
| PSA | 142.96000 |
| LogP | 1.96530 |
| Appearance of Characters | white |
| Index of Refraction | 1.6500 (estimate) |
| Storage condition | -20°C Freezer |
| Water Solubility | H2O: 25 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 + H311 + H331-H361 |
| Precautionary Statements | Missing Phrase - N15.00950417-P261-P280-P302 + P352 + P312-P304 + P340 + P312-P403 + P233 |
| Hazard Codes | T |
| Risk Phrases | R23/24/25;R62 |
| Safety Phrases | S36/37/39-S45 |
| RIDADR | UN 1544 |
| WGK Germany | 3 |
| RTECS | KE5500000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
Methylergometrine: adverse effects in breastfed infants.
Prescrire Int. 23(148) , 102, (2014)
|
|
|
[Reflections of an expert committee of the French Society of Cardiology on the use of methylergometrine maleate (Methergin) for the detection of abnormal coronary vasomotricity].
Arch. Mal. Coeur Vaiss. 88(2) , 247-53, (1995)
|
|
|
Oral methylergonovine maleate for refractory migraine and cluster headache prevention.
Headache 53(2) , 378-81, (2013)
|
| (6aR,9R)-N-[(2S)-1-hydroxybutan-2-yl]-7-methyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide,(Z)-but-2-enedioic acid |