N-Me-DL-Ala-OH.HCl
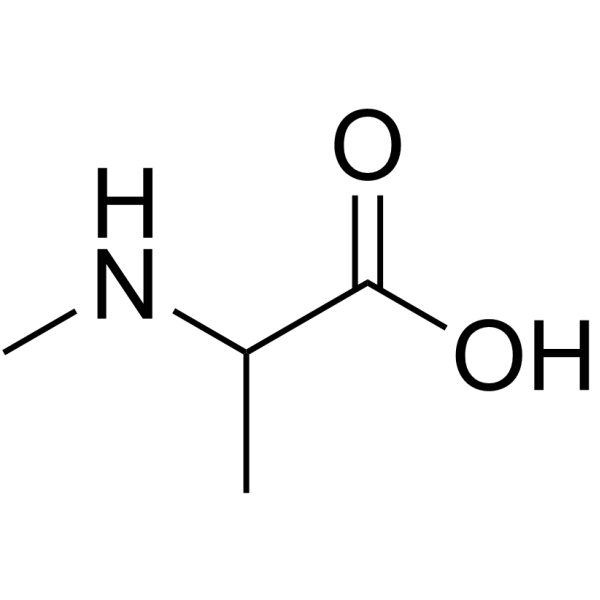
N-Me-DL-Ala-OH.HCl structure
|
Common Name | N-Me-DL-Ala-OH.HCl | ||
|---|---|---|---|---|
| CAS Number | 600-21-5 | Molecular Weight | 103.120 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 190.1±23.0 °C at 760 mmHg | |
| Molecular Formula | C4H9NO2 | Melting Point | 317CºC | |
| MSDS | Chinese USA | Flash Point | 68.8±22.6 °C | |
Use of N-Me-DL-Ala-OH.HClH-N-Me-DL-Ala-OH is an alanine derivative[1]. |
| Name | 2-(methylamino)propanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | H-N-Me-DL-Ala-OH is an alanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 190.1±23.0 °C at 760 mmHg |
| Melting Point | 317CºC |
| Molecular Formula | C4H9NO2 |
| Molecular Weight | 103.120 |
| Flash Point | 68.8±22.6 °C |
| Exact Mass | 103.063332 |
| PSA | 49.33000 |
| LogP | -0.44 |
| Vapour Pressure | 0.2±0.7 mmHg at 25°C |
| Index of Refraction | 1.436 |
| Storage condition | Store at RT. |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2922499990 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
1H-NMR and 13C-NMR investigation of complexes of Mn2+ with ocytocin analogues in (2H6)dimethylsulfoxide.
Eur. J. Biochem. 240(1) , 118-24, (1996) Several ocytocin analogues were synthesised by substitution of the Pro residue with sarcosine or N-methylalanine, the glutamine residue with threonine and one of the cysteines with 2-mercaptopropionic... |
|
|
Transport of L-alanine in cultured human fibroblasts: evidence for two kinetically distinguishable systems.
J. Cell Physiol. 109(1) , 37-43, (1981) The transport of L-alanine in human diploid fibroblasts was investigated. Transport measurements were performed on subcultures between the third and eighth passages with subconfluent cells growing on ... |
|
|
Monomeric sarcosine oxidase: 2. Kinetic studies with sarcosine, alternate substrates, and a substrate analogue.
Biochemistry 39 , 8825-9, (2000) Monomeric sarcosine oxidase (MSOX) is a flavoenzyme that catalyzes the oxidative demethylation of sarcosine (N-methylglycine) to yield glycine, formaldehyde, and hydrogen peroxide. MSOX can oxidize ot... |
| 2-(methylamino)propionic acid |
| 2-(Methylamino)propanoic acid |
| 2-(N-methylamino)propionic acid |
| N-Me-DL-Ala-OH.HCl |
| Alanine, N-methyl- |
| N-|A-Methyl-DL-alanine |
| L-Alanine,N-methyl |
| N-Methyl-D-Alanine |
| N-Methyl-DL-alanine |
| MFCD00063136 |
| N-Methylalanine |
| N-Methyl-Alanine |
| Alanine,N-methyl-,L |
 CAS#:101692-94-8
CAS#:101692-94-8 CAS#:127-17-3
CAS#:127-17-3 CAS#:103-67-3
CAS#:103-67-3 CAS#:74-89-5
CAS#:74-89-5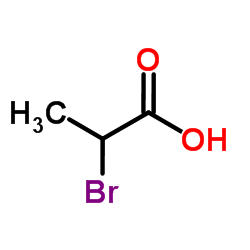 CAS#:598-72-1
CAS#:598-72-1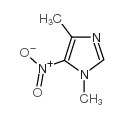 CAS#:57658-79-4
CAS#:57658-79-4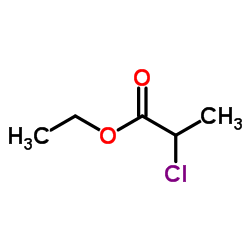 CAS#:535-13-7
CAS#:535-13-7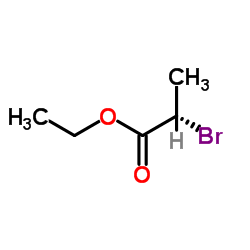 CAS#:535-11-5
CAS#:535-11-5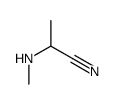 CAS#:16752-54-8
CAS#:16752-54-8 CAS#:50-00-0
CAS#:50-00-0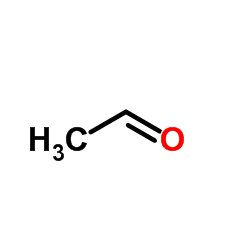 CAS#:75-07-0
CAS#:75-07-0 CAS#:302-72-7
CAS#:302-72-7 CAS#:124-38-9
CAS#:124-38-9 CAS#:64-19-7
CAS#:64-19-7 CAS#:90-82-4
CAS#:90-82-4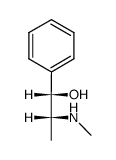 CAS#:90-81-3
CAS#:90-81-3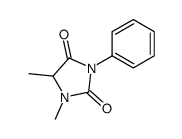 CAS#:2221-10-5
CAS#:2221-10-5 CAS#:102-07-8
CAS#:102-07-8
