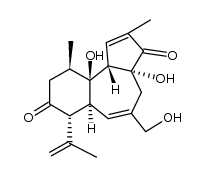
Prostratin
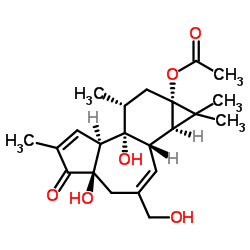
Prostratin structure
|
Common Name | Prostratin | ||
|---|---|---|---|---|
| CAS Number | 60857-08-1 | Molecular Weight | 390.470 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 550.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H30O6 | Melting Point | 216-219℃ | |
| MSDS | Chinese USA | Flash Point | 188.7±23.6 °C | |
Use of ProstratinProstratin, a natural terpenoid compound, is a PKC activator, with a Ki of 12.5 nM and shows inhibitory effect on HIV-1. |
| Name | 12-deoxyphorbol 13-acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Prostratin, a natural terpenoid compound, is a PKC activator, with a Ki of 12.5 nM and shows inhibitory effect on HIV-1. |
|---|---|
| Related Catalog | |
| Target |
PKC:12.5 nM (Ki) HIV-1 |
| In Vitro | Prostratin 抑制 [3H]PDBu 与 CEM 细胞的结合,Ki 为 210 nM[1]。 Prostratin (125-1000 nM) 剂量依赖性地抑制急性髓性白血病 (AML) 细胞系 (HL-60、NB4 和 U937 细胞) 的生长。Prostratin (125-100 nM) 诱导 AML 细胞的 G1 期阻滞并影响 HL-60 细胞中的细胞周期相关分子 (pRb 磷酸化、CDK 和 p21)。Prostratin 还通过激活 PKC 导致 AML 细胞系分化。此外,MEK/ERK/MAP 信号通路的 PKC 依赖性激活需要 Prostratin 诱导的分化[2]。 Prostratin 诱导需要 PKD3 活性形式的 HIV-1 转录激活。Prostratin 还通过新型 PKC 亚家族的 PKCε 激活 PKD3[3]。 Cell Viability Assay[2] Cell Line: HL-60, NB4 and U937 cells Concentration: 125 nM, 250 nM, 500 nM, 1000 nM Incubation Time: 24 hours, 48 hours, 72 hours Result: Dose-dependently inhibited the growth of acute myeloid leukemia (AML) cell lines. Cell Cycle Analysis[2] Cell Line: HL-60, NB4 and U937 cells Concentration: 125 nM, 250 nM, 500 nM, 1000 nM Incubation Time: 24 hours Result: Induced a G0/G1 phase accumulation in a concentration-dependent manner. Western Blot Analysis[2] Cell Line: HL-60 cells Concentration: 125 nM, 250 nM, 500 nM, 1000 nM Incubation Time: 24 hours Result: Affected the cell-cycle-related molecules (pRb phosphorylation, CDKs, and p21) in HL-60 cells. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 550.5±50.0 °C at 760 mmHg |
| Melting Point | 216-219℃ |
| Molecular Formula | C22H30O6 |
| Molecular Weight | 390.470 |
| Flash Point | 188.7±23.6 °C |
| Exact Mass | 390.204254 |
| PSA | 104.06000 |
| LogP | 1.84 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.600 |
| InChIKey | BOJKFRKNLSCGHY-HXGSDTCMSA-N |
| SMILES | CC(=O)OC12CC(C)C3(O)C(C=C(CO)CC4(O)C(=O)C(C)=CC43)C1C2(C)C |
| Storage condition | ?20°C |
| Water Solubility | Soluble in DMSO at 30mg/ml |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~90% 
Prostratin CAS#:60857-08-1 |
| Literature: The Board of Trustees of the Leland Stanford Junior University Patent: US2009/187046 A1, 2009 ; Location in patent: Page/Page column 11 ; |
|
~35% 
Prostratin CAS#:60857-08-1 |
| Literature: The Board of Trustees of the Leland Stanford Junior University Patent: US2009/187046 A1, 2009 ; Location in patent: Page/Page column 12-13 ; |
|
~% 
Prostratin CAS#:60857-08-1 |
| Literature: Natural Product Research, , vol. 27, # 16 p. 1459 - 1462 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
IFI44 suppresses HIV-1 LTR promoter activity and facilitates its latency.
Virology 481 , 142-50, (2015) IFI44 is an interferon-alfa inducible protein, and is associated with infection of several viruses. However, IFI44 elicits minimal antiviral effects on these viruses, and its exact role is still unkno... |
|
|
Sulfonation pathway inhibitors block reactivation of latent HIV-1.
Virology 471-473 , 1-12, (2014) Long-lived pools of latently infected cells are a significant barrier to the development of a cure for HIV-1 infection. A better understanding of the mechanisms of reactivation from latency is needed ... |
|
|
Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection.
PLoS ONE 4(6) , e6093, (2009) The persistence of transcriptionally silent but replication-competent HIV-1 reservoirs in Highly Active Anti-Retroviral Therapy (HAART)-treated infected individuals, represents a major hurdle to virus... |
| (1aR,1bS,4aR,7aS,7bR,8R,9aS)-4a,7b-Dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo-1,1a,1b,4,4a,5,7a,7b,8,9-decahydro-9aH-cyclopropa[3,4]benzo[1,2-e]azulen-9a-yl acetate |
| 13-O-Acetyl-12-deoxyphorbol |
| 12-Deoxyphorbol-13-acetate |
| Stillingia Facto |
| 5H-Cyclopropa[3,4]benz[1,2-e]azulen-5-one, 9a-(acetyloxy)-1,1a,1b,4,4a,7a,7b,8,9,9a-decahydro-4a,7b-dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-, (1aR,1bS,4aR,7aS,7bR,8R,9aS)- |
| 12-Deoxyphorbol 13-Acetate,dPAc |
| 13-O-Acetylphorbol |
| Stillingia factor S7 |
| SA 101A |
| prostratin |
| 12-deoxyphorbal-13-acetate |
![(3aR,3bS,6aR,9aS,9bR,10R,11aS)-6a,9b-dihydroxy-5-(hydroxymethyl)-3,3,8,10-tetramethyl-7-oxo-3,3a,3b,6,6a,7,9a,9b,10,11-decahydro-11aH-azuleno[5,4-e]indazol-11a-yl acetate structure](https://image.chemsrc.com/caspic/476/1035455-44-7.png)