tectoridin
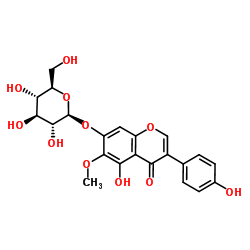
tectoridin structure
|
Common Name | tectoridin | ||
|---|---|---|---|---|
| CAS Number | 611-40-5 | Molecular Weight | 462.404 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 798.1±60.0 °C at 760 mmHg | |
| Molecular Formula | C22H22O11 | Melting Point | 261.8-263.2ºC | |
| MSDS | Chinese USA | Flash Point | 279.7±26.4 °C | |
Use of tectoridinTectoridin is a isoflavone isolated from Maackia amurensis. Tectoridin is a phytoestrogen and activates estrogen and thyroid hormone receptors. Tectoridin exerts the estrogenic effects via ER-dependent genomic pathway and GPR30-dependent nongenomic pathway[1][2]. |
| Name | tectoridin |
|---|---|
| Synonym | More Synonyms |
| Description | Tectoridin is a isoflavone isolated from Maackia amurensis. Tectoridin is a phytoestrogen and activates estrogen and thyroid hormone receptors. Tectoridin exerts the estrogenic effects via ER-dependent genomic pathway and GPR30-dependent nongenomic pathway[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Tectoridin scarcely binds to ER alpha as compared to 17beta-estradiol and genistein[2]. Tectoridin induceds potent estrogenic effects, namely recovery of the population of cells in the S-phase after serum starvation, transactivation of the estrogen response element, and induction of MCF-7 cell proliferation[2]. Tectoridin induces estrogenic effect, and this effect is severely abrogated by treatment with U0126 ( MEK1/2 inhibitor). Tectoridin promotes phosphorylation of ERK1/2, but does not affect phosphorylation of ER alpha at Ser (118). It also increases cellular accumulation of cAMP[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 798.1±60.0 °C at 760 mmHg |
| Melting Point | 261.8-263.2ºC |
| Molecular Formula | C22H22O11 |
| Molecular Weight | 462.404 |
| Flash Point | 279.7±26.4 °C |
| Exact Mass | 462.116211 |
| PSA | 179.28000 |
| LogP | 0.29 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.695 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | C |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | DJ3090000 |
| HS Code | 29389090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
Tectorigenin inhibits IFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells.
Arch. Pharm. Res. 31(11) , 1447-56, (2008) Tectorigenin (Tg) and tectoridin (Td) are the major compounds isolated from the rhizomes of iridaceous plant Belamcanda chinensis which is well known as a chinese traditional medicine for the treatmen... |
|
|
Tectoridin, a poor ligand of estrogen receptor alpha, exerts its estrogenic effects via an ERK-dependent pathway.
Mol. Cells 27(3) , 351-7, (2009) Phytoestrogens are the natural compounds isolated from plants, which are structurally similar to animal estrogen, 17beta-estradiol. Tectoridin, a major isoflavone isolated from the rhizome of Belamcan... |
|
|
Microbial transformation and bioactivation of isoflavones from Pueraria flowers by human intestinal bacterial strains.
J. Nat. Med. 63(3) , 254-60, (2009) The flowers from Pueraria, which are called Puerariae Flos, have been used since ancient times for recovery from alcohol intoxication. We elucidated the microbial transformation of the main isoflavone... |
| 4H-1-Benzopyran-4-one, 7-(β-D-glucopyranosyloxy)-5-hydroxy-3-(4-hydroxyphenyl)-6-methoxy- |
| Tectorigenin 7-glucoside |
| 4',5-Dihydro-6-methoxy-7-(o-glucoside)isoflavone |
| 5-Hydroxy-3-(4-hydroxyphenyl)-6-methoxy-4-oxo-4H-chromen-7-yl β-D-glucopyranoside |
| Shekanin |
| tectoridin |
| Tectoridin (7CI,8CI) |
| 5-hydroxy-3-(4-hydroxyphenyl)-6-methoxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one |
 CAS#:2345-17-7
CAS#:2345-17-7
