Chloroquinoline
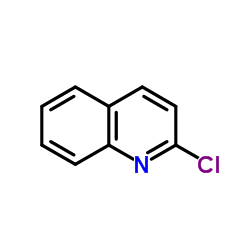
Chloroquinoline structure
|
Common Name | Chloroquinoline | ||
|---|---|---|---|---|
| CAS Number | 612-62-4 | Molecular Weight | 163.604 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 267.3±13.0 °C at 760 mmHg | |
| Molecular Formula | C9H6ClN | Melting Point | 34-37 °C(lit.) | |
| MSDS | USA | Flash Point | 141.2±5.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2-Chloroquinoline |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 267.3±13.0 °C at 760 mmHg |
| Melting Point | 34-37 °C(lit.) |
| Molecular Formula | C9H6ClN |
| Molecular Weight | 163.604 |
| Flash Point | 141.2±5.4 °C |
| Exact Mass | 163.018875 |
| PSA | 12.89000 |
| LogP | 2.71 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.652 |
| InChIKey | OFUFXTHGZWIDDB-UHFFFAOYSA-N |
| SMILES | Clc1ccc2ccccc2n1 |
| Water Solubility | Insoluble |
CHEMICAL IDENTIFICATION
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | VB2320000 |
| HS Code | 2933499090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Antiprotozoal activity of chloroquinoline based chalcones.
Eur. J. Med. Chem. 46 , 1897-905, (2011) A new series of chloroquinoline based chalcones were synthesized and evaluated for in vitro antiamoebic and antimalarial activities. The results showed that out of fifteen compounds, four were found t... |
|
|
Ruthenium-catalyzed cyclization of anilides with substituted propiolates or acrylates: an efficient route to 2-quinolinones.
Org. Lett. 16(13) , 3568-71, (2014) A Ru-catalyzed cyclization of anilides with propiolates or acrylates affording 2-quinolinones having diverse functional groups in good to excellent yields is described. Later, 2-quinolinones were conv... |
|
|
Chemoenzymatic synthesis of chiral 2,2'-bipyridine ligands and their N-oxide derivatives: applications in the asymmetric aminolysis of epoxides and asymmetric allylation of aldehydes.
Org. Biomol. Chem. 8(5) , 1081-90, (2010) A series of enantiopure 2,2'-bipyridines have been synthesised from the corresponding cis-dihydrodiol metabolites of 2-chloroquinolines. Several of the resulting hydroxylated 2,2'-bipyridines were fou... |
| 2-Chloroquinoline 1GR |
| 2CQ |
| Chloroquinoline |
| 2-Chlor-chinolin |
| 2-chloro-quinoline |
| Quinoline, 2-chloro- |
| EINECS 210-317-8 |
| quinolyl chloride |
| Quinoline2-chloro |
| 2-chloro-quinolin |
| 2-Chloroquinoline |
| MFCD00006741 |
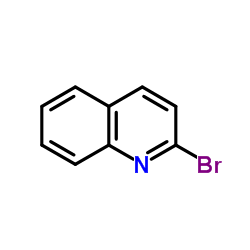 CAS#:2005-43-8
CAS#:2005-43-8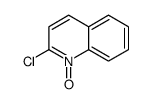 CAS#:2423-68-9
CAS#:2423-68-9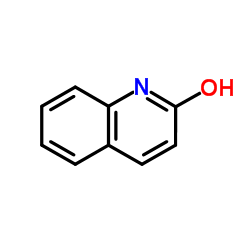 CAS#:59-31-4
CAS#:59-31-4 CAS#:2889-13-6
CAS#:2889-13-6 CAS#:3867-18-3
CAS#:3867-18-3 CAS#:503-38-8
CAS#:503-38-8 CAS#:19591-17-4
CAS#:19591-17-4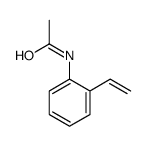 CAS#:29124-68-3
CAS#:29124-68-3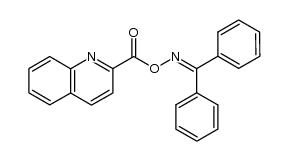 CAS#:115975-18-3
CAS#:115975-18-3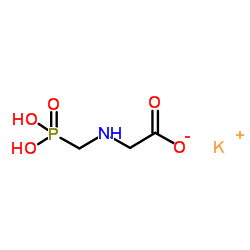 CAS#:1613-37-2
CAS#:1613-37-2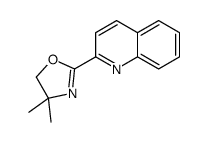 CAS#:109660-13-1
CAS#:109660-13-1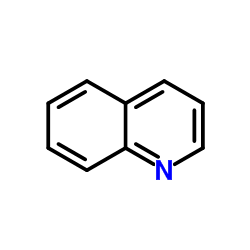 CAS#:91-22-5
CAS#:91-22-5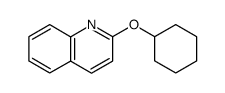 CAS#:80754-07-0
CAS#:80754-07-0 CAS#:108-94-1
CAS#:108-94-1 CAS#:580-22-3
CAS#:580-22-3 CAS#:700-79-8
CAS#:700-79-8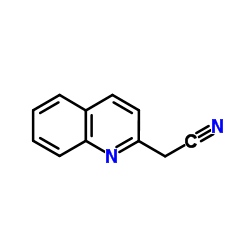 CAS#:14068-28-1
CAS#:14068-28-1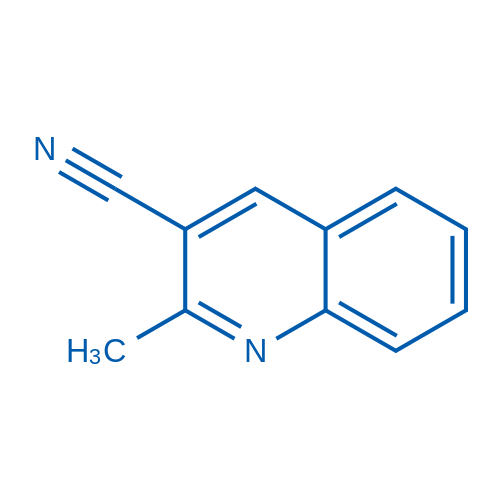 CAS#:72248-92-1
CAS#:72248-92-1 CAS#:91301-03-0
CAS#:91301-03-0 CAS#:4491-33-2
CAS#:4491-33-2
