Betaxolol
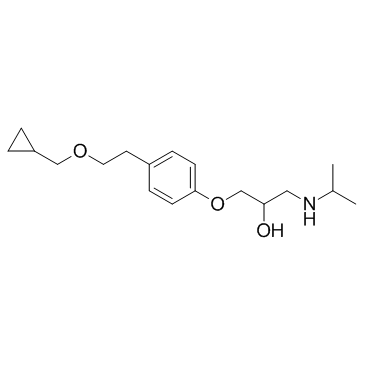
Betaxolol structure
|
Common Name | Betaxolol | ||
|---|---|---|---|---|
| CAS Number | 63659-18-7 | Molecular Weight | 307.43 | |
| Density | 1.067 g/cm3 | Boiling Point | 448ºC at 760 mmHg | |
| Molecular Formula | C18H29NO3 | Melting Point | 61-63°C | |
| MSDS | N/A | Flash Point | 224.7ºC | |
Use of BetaxololBetaxolol is a selective beta1 adrenergic receptor blocker used in the treatment of hypertension and glaucoma.Target: Beta1 Adrenergic ReceptorBetaxolol is a cardioselective beta-adrenergic receptor blocking agent. Betaxolol (5 mg/kg via i.p. injection) was administered at 24 and then 44 h following the final chronic cocaine administration. Animals treated with betaxolol during cocaine withdrawal exhibited a significant attenuation of anxiety-like behavior characterized by increased time spent in the open arms and increased entries into the open arms compared to animals treated with only saline during cocaine withdrawal. Betaxolol did not produce anxiolytic-like effects in control animals treated chronically with saline [1]. Betaxolol produces less systemic beta 2- and possibly beta 1-adrenergic receptor blockade than either timolol or levobunolol. Betaxolol may be relatively safer to use in patients with reactive airway disease than either timolol or levobunolol [2]. |
| Name | betaxolol |
|---|---|
| Synonym | More Synonyms |
| Description | Betaxolol is a selective beta1 adrenergic receptor blocker used in the treatment of hypertension and glaucoma.Target: Beta1 Adrenergic ReceptorBetaxolol is a cardioselective beta-adrenergic receptor blocking agent. Betaxolol (5 mg/kg via i.p. injection) was administered at 24 and then 44 h following the final chronic cocaine administration. Animals treated with betaxolol during cocaine withdrawal exhibited a significant attenuation of anxiety-like behavior characterized by increased time spent in the open arms and increased entries into the open arms compared to animals treated with only saline during cocaine withdrawal. Betaxolol did not produce anxiolytic-like effects in control animals treated chronically with saline [1]. Betaxolol produces less systemic beta 2- and possibly beta 1-adrenergic receptor blockade than either timolol or levobunolol. Betaxolol may be relatively safer to use in patients with reactive airway disease than either timolol or levobunolol [2]. |
|---|---|
| Related Catalog | |
| References |
[2]. Lesar, T.S., Comparison of ophthalmic beta-blocking agents. Clin Pharm, 1987. 6(6): p. 451-63. |
| Density | 1.067 g/cm3 |
|---|---|
| Boiling Point | 448ºC at 760 mmHg |
| Melting Point | 61-63°C |
| Molecular Formula | C18H29NO3 |
| Molecular Weight | 307.43 |
| Flash Point | 224.7ºC |
| PSA | 50.72000 |
| LogP | 2.78430 |
| InChIKey | NWIUTZDMDHAVTP-UHFFFAOYSA-N |
| SMILES | CC(C)NCC(O)COc1ccc(CCOCC2CC2)cc1 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26 |
| WGK Germany | 3 |
| RTECS | GW2967200 |
|
~86% 
Betaxolol CAS#:63659-18-7 |
| Literature: Joshi, Ramesh Anna; Murugan, Muthukrishnan; Garud, Dinesh Ramesh; Borikar, Sanjay Pandurang; Gurjar, Mukund Keshav Patent: US2006/94903 A1, 2006 ; Location in patent: Page/Page column 5 ; |
|
~92% 
Betaxolol CAS#:63659-18-7 |
| Literature: Di Bono; Scilimati Synthesis, 1995 , # 6 p. 699 - 702 |
|
~% 
Betaxolol CAS#:63659-18-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 6 p. 1003 - 1011 |
|
~% 
Betaxolol CAS#:63659-18-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 6 p. 1003 - 1011 |
|
~% 
Betaxolol CAS#:63659-18-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 6 p. 1003 - 1011 |
|
~% 
Betaxolol CAS#:63659-18-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 6 p. 1003 - 1011 |
| Precursor 7 | |
|---|---|
| DownStream 2 | |
| (±)-1-(p-(2-(Cyclopropylmethoxy)ethyl)phenoxy)-3-(isopropylamino)-2-propanol Hydrochloride |
| 1-{4-[2-(cyclopropylmethoxy)ethyl]phenoxy}-3-[(1-methylethyl)amino]propan-2-ol hydrochloride |
| Betaxolol |
| MFCD00242958 |
| (+/-)-1-{p-[2-(cyclopropylmethoxy) ethyl] phenoxy}-3-isopropylamino-2-propanol |
| 1-{4-[2-(Cyclopropylmethoxy)ethyl]phenoxy}-3-[(1-methylethyl)amino]propan-2-olhydrochlorid |
| Betaxololum |
| 2-Propanol, 1-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-[(1-methylethyl)amino]-, hydrochloride (1:1) |
| 1-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol |
| 1-{4-[2-(Cyclopropylmethoxy)ethyl]phenoxy}-3-(isopropylamino)-2-propanol hydrochloride (1:1) |
| Betaxolol [INN:BAN] |
| 1-{4-[2-(cyclopropylmethoxy)ethyl]phenoxy}-3-(propan-2-ylamino)propan-2-ol hydrochloride (1:1) |
| Betaxolol hydrochloride |
| Kerlone |
| 1-{4-[2-(cyclopropylméthoxy)éthyl]phénoxy}-3-[(1-méthyléthyl)amino]propan-2-ol chlorhydrate |
| Betaxolol (TN) |
| 1-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-(propan-2-ylamino)propan-2-ol |
| 1-{4-[2-(Cyclopropylmethoxy)-ethyl]-phenoxy}-3-isopropylamino-propan-2-ol |
| 1-{4-[2-(Cyclopropylmethoxy)ethyl]phenoxy}-3-(isopropylamino)propan-2-ol hydrochloride (1:1) |
| Betoptic |
| Betaxololum [INN-Latin] |
| Betazolol |
| 2-propanol, 1-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-[(1-methylethyl)amino]-, hydrochloride |
| UNII:6X97D2XT0O |

![1-[4-(2-Hydroxyethyl)phenoxy]-2,3-epoxypropane structure](https://image.chemsrc.com/caspic/251/63659-17-6.png)
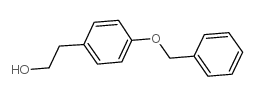

![4-[2-(Cyclopropylmethoxy)ethyl]phenol structure](https://image.chemsrc.com/caspic/289/63659-16-5.png)
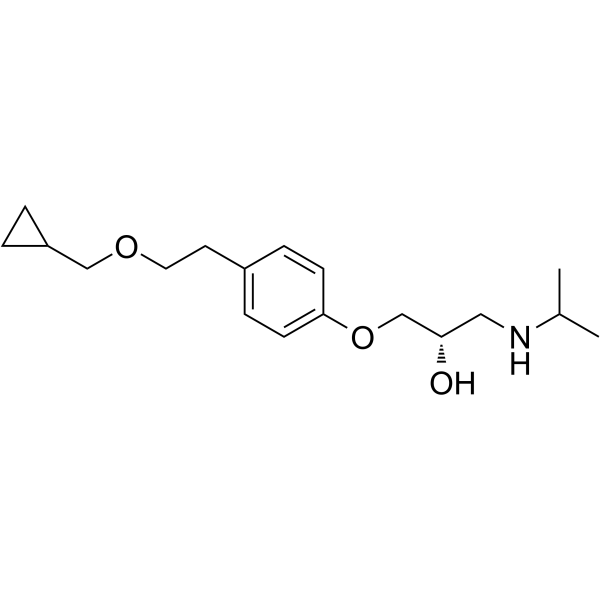 CAS#:93221-48-8
CAS#:93221-48-8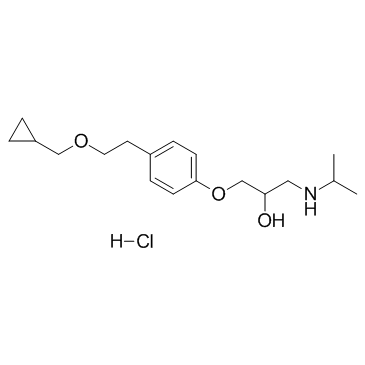 CAS#:63659-19-8
CAS#:63659-19-8