Desonide
Modify Date: 2025-08-20 13:36:04
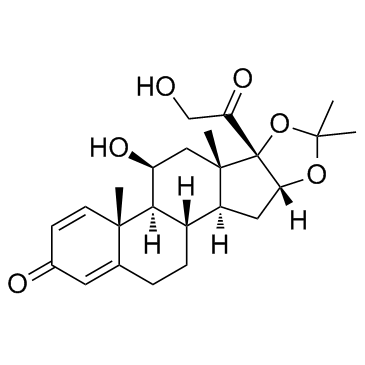
Desonide structure
|
Common Name | Desonide | ||
|---|---|---|---|---|
| CAS Number | 638-94-8 | Molecular Weight | 416.507 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 580.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H32O6 | Melting Point | 274 - 275ºC | |
| MSDS | N/A | Flash Point | 196.9±23.6 °C | |
Use of DesonideDesonide is a nonfluorinated corticosteroid anti-inflammatory agent used topically for dermatoses.Target: Glucocorticoid ReceptorDesonide is a low-potency topical corticosteroid that has been used for decades in the treatment of steroid-responsive dermatoses [1]. Desonide induced significant colorimetric improvement compared with placebo. A good to excellent response was achieved in 30% for desonide, and 6% for placebo. Decreased pigmentation in the desonide-treated axillae was associated with recovery of disruption at the basal membrane. Desonide showed depigmenting properties in women with axillary hyperpigmentation [2]. Given the favorable safety profile of all other desonide preparations and their utility as a low potency corticosteroid, desonide foam promises to be a useful addition to the armamentarium, when other desonide vehicles might be less acceptable [3]. |
| Name | desonide |
|---|---|
| Synonym | More Synonyms |
| Description | Desonide is a nonfluorinated corticosteroid anti-inflammatory agent used topically for dermatoses.Target: Glucocorticoid ReceptorDesonide is a low-potency topical corticosteroid that has been used for decades in the treatment of steroid-responsive dermatoses [1]. Desonide induced significant colorimetric improvement compared with placebo. A good to excellent response was achieved in 30% for desonide, and 6% for placebo. Decreased pigmentation in the desonide-treated axillae was associated with recovery of disruption at the basal membrane. Desonide showed depigmenting properties in women with axillary hyperpigmentation [2]. Given the favorable safety profile of all other desonide preparations and their utility as a low potency corticosteroid, desonide foam promises to be a useful addition to the armamentarium, when other desonide vehicles might be less acceptable [3]. |
|---|---|
| Related Catalog | |
| References |
[3]. Parish, D. and N. Scheinfeld, Desonide foam: a review. Drugs Today (Barc), 2008. 44(1): p. 55-62. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 580.1±50.0 °C at 760 mmHg |
| Melting Point | 274 - 275ºC |
| Molecular Formula | C24H32O6 |
| Molecular Weight | 416.507 |
| Flash Point | 196.9±23.6 °C |
| Exact Mass | 416.219879 |
| PSA | 93.06000 |
| LogP | 2.62 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.598 |
| InChIKey | WBGKWQHBNHJJPZ-LECWWXJVSA-N |
| SMILES | CC1(C)OC2CC3C4CCC5=CC(=O)C=CC5(C)C4C(O)CC3(C)C2(C(=O)CO)O1 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2937210000 |
|
~% 
Desonide CAS#:638-94-8 |
| Literature: Journal of the American Chemical Society, , vol. 81, p. 4573 |
| Precursor 1 | |
|---|---|
| DownStream 2 | |
| HS Code | 2937210000 |
|---|
| Prednacinolone |
| (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one |
| 16a,17a-Isopropylidenedioxyprednisolone |
| 2H-naphth[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one, 4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-, (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)- |
| Desowen |
| 2H-Naphth[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one, 4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-5-hydroxy-6b-(2-hydroxyacetyl)-4a,6a,8,8-tetramethyl-, (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)- |
| Locapred |
| Desonide |
| (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-5-hydroxy-6b-(hydroxyacétyl)-4a,6a,8,8-tétraméthyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodécahydro-2H-naphto[2',1':4,5]indéno[1,2-d][1,3]dioxol-2-one |
| Sterax |
| 11b,16a,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione Cyclic 16,17-Acetal with Acetone |
| 16a-Hydroxy-D1-hydrocortisone-16a,17a-acetonide |
| 16a-Hydroxyprednisolone-16a,17-acetonide |
| Steroderm |
| 16a-Hydroxyprednisolone Acetonide |
| TRIDESILON |
| desonidum [INN_la] |
| MFCD00866151 |
| 11β,16α,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone |
| (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-5-Hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-on |
| (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-6b-Glycoloyl-5-hydroxy-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one |
| 11b,21-Dihydroxy-16a,17-isopropylidenedioxy-1,4-pregnadiene-3,20-dione |
| Topifug |
| d-2083 |
| EINECS 211-351-6 |
| 11,21-Dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione |
| Budesonide Impurity 6 |

 CAS#:51372-29-3
CAS#:51372-29-3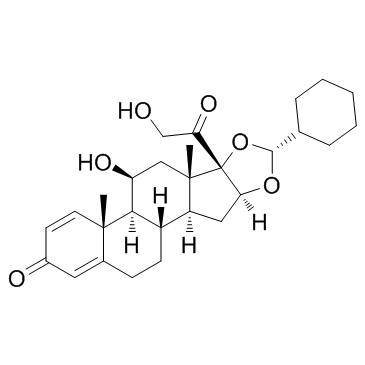 CAS#:161115-59-9
CAS#:161115-59-9
