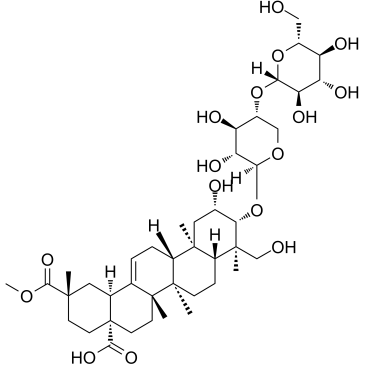
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
RK0177530
-
CHEMICAL NAME :
-
Olean-12-ene-28,29-dioic acid, 2,23-dihydroxy-3-((4-O-beta-D-glucopyranosyl-beta-D- xylopyranosyl)oxy)-, 29-methyl ester, (2-beta,3-beta,4-alpha,20-beta)-
-
CAS REGISTRY NUMBER :
-
65497-07-6
-
LAST UPDATED :
-
198706
-
DATA ITEMS CITED :
-
4
-
MOLECULAR FORMULA :
-
C42-H66-O16
-
MOLECULAR WEIGHT :
-
827.08
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
42300 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
SYHJAM Saengyak Hakhoechi. Journal of the Society of Pharmacognosy. (Hanguk Saengyak Hakhoe, c/o Natural Products Institute, Seoul National Univ., 28 Yunkeon-Dong, Chong-ro-ku, Seoul 110, Korea) 1970- Volume(issue)/page/year: 10,73,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1200 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Skin and Appendages - hair Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
SHZAAY Shoyakugaku Zasshi. Journal of Pharmacognosy. (Nippon Shoyaku Gakkai, c/o Nippon Gakkai Jimu Senta, 2-4-16 Yayoi, Bunkyo-ku, Tokyo 113, Japan) V.6- 1952- Volume(issue)/page/year: 33,111,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
486 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Skin and Appendages - hair Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
SHZAAY Shoyakugaku Zasshi. Journal of Pharmacognosy. (Nippon Shoyaku Gakkai, c/o Nippon Gakkai Jimu Senta, 2-4-16 Yayoi, Bunkyo-ku, Tokyo 113, Japan) V.6- 1952- Volume(issue)/page/year: 33,111,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
23600 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
SYHJAM Saengyak Hakhoechi. Journal of the Society of Pharmacognosy. (Hanguk Saengyak Hakhoe, c/o Natural Products Institute, Seoul National Univ., 28 Yunkeon-Dong, Chong-ro-ku, Seoul 110, Korea) 1970- Volume(issue)/page/year: 10,73,1979
|