65497-07-6
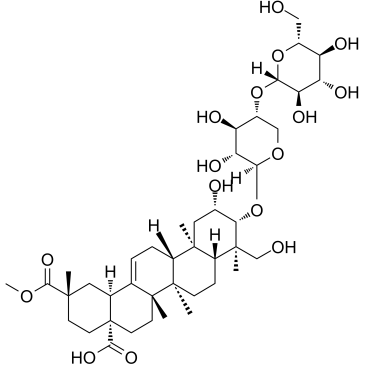
| Name | Esculentoside A |
|---|---|
| Synonyms |
Esculentoside
4-O-(2,23,28-Trihydroxy-29-methoxy-28,29-dioxoolean-12-en-3-yl)pentopyranosyl hexopyranoside EsculentosideA (2β,3β)-3-{[4-O-(β-D-Glucopyranosyl)-β-D-xylopyranosyl]oxy}-2,23-dihydroxy-30-methoxy-30-oxoolean-12-en-29-oic acid Phytolacaponin E Hexopyranoside, 4-O-(2,23,28-trihydroxy-29-methoxy-28,29-dioxoolean-12-en-3-yl)pentopyranosyl |
| Description | Esculentoside A (EsA), a kind of triterpene saponin isolated from roots of Phytolacca esculenta[1].Esculentoside A (EsA) possesses anti-inflammatory activity in acute and chronic experimental models[2], has selective inhibitory activity towards cyclooxygenase-2 (COX-2)[1].Esculentoside A (EsA) suppresses inflammatory responses in LPS-induced acute lung injury (ALI) through inhibition of the nuclear factor kappa B (NF-ΚB) and mitogen activated protein kinase (MAPK) signaling pathways[3]. |
|---|---|
| Related Catalog | |
| In Vitro | Esculentoside A (0-10 μM; 24 hours) reduced the release of TNF concentration in primed macrophages [4]. |
| In Vivo | Esculentoside A (EsA) (intraperitoneal injection; 20 mg/kg; once a day; 4 weeks) plays significant roles in the treatment of BXSB mice through modulation of inflammatory cytokines, inhibition of renal cell proliferation and induction of apoptosis [2]. Esculentoside A (EsA) (injected intraperitoneally; 5, 10 and 20 mg/kg; once a day; 7 days) dose-dependently decreases the TNF, IL-1 and IL-6 levels in the sera of mice following LPS challenge[4]. Animal Model: BXSB mice[2] Dosage: 20 mg/kg Administration: Intraperitoneal injection; 20 mg/kg; once a day; 4 weeks Result: Alleviated the renal damage of LN. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 935.8±65.0 °C at 760 mmHg |
| Molecular Formula | C42H66O16 |
| Molecular Weight | 826.964 |
| Flash Point | 275.1±27.8 °C |
| Exact Mass | 826.435059 |
| PSA | 262.36000 |
| LogP | 3.01 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.620 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | 24/25 |
|---|