Xanthohumol
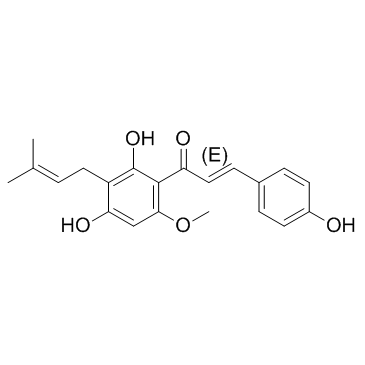
Xanthohumol structure
|
Common Name | Xanthohumol | ||
|---|---|---|---|---|
| CAS Number | 6754-58-1 | Molecular Weight | 354.396 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 576.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H22O5 | Melting Point | 157-159ºC | |
| MSDS | Chinese USA | Flash Point | 203.4±23.6 °C | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of XanthohumolXanthohumol is one of the principal flavonoids isolated from hops, the inhibitor of diacylglycerol acetyltransferase (DGAT), COX-1 and COX-2, and shows anti-cancer and anti-angiogenic activities. |
| Name | xanthohumol |
|---|---|
| Synonym | More Synonyms |
| Description | Xanthohumol is one of the principal flavonoids isolated from hops, the inhibitor of diacylglycerol acetyltransferase (DGAT), COX-1 and COX-2, and shows anti-cancer and anti-angiogenic activities. |
|---|---|
| Related Catalog | |
| In Vitro | Xanthohumol significantly attenuates ADP-induced blood platelet aggregation, and significantly reduces the expression of fibrinogen receptor (activated form of GPIIbIIIa) on platelets' surface[1]. Xanthohumol (5-50 nM) reduces the frequency of spontaneously occurring Ca2+ sparks and Ca2+ waves in control myocytes and in cells subjected to Ca2+ overload caused by: (1) exposure to low K+ solutions, (2) periods of high frequency electrical stimulation, (3) exposures to isoproterenol or (4) caffeine. Xanthohumol (50-100 nM) reduces the rate of relaxation of electrically- or caffeine-triggered Ca2+ transients, without suppressing ICa, but this effect is small and reversed by isoproterenol at physiological temperatures. Xanthohumol also suppresses the Ca2+ content of the SR, and its rate of recirculation[2]. Treatment of endothelial cells with Xanthohumol leads to increased AMPK phosphorylation and activity. Functional studies using biochemical approaches confirm that AMPK mediates Xanthohumol anti-angiogenic activity. AMPK activation by Xanthohumol is mediated by CAMMKβ, but not LKB1. Analysis of the downstream mechanisms shows that Xanthohumol-induced AMPK activation reduces nitric oxide (NO) levels in endothelial cells by decreasing eNOS phosphorylation. Finally, AKT pathway is inactivated by Xanthohumol as part of its anti-angiogenic activity, but independently from AMPK, suggesting that these two signaling pathways proceed autonomously[3]. Xanthohumol significantly reduces cell viability and induces apoptosis via pro-caspase-3/8 cleavage and poly(ADP ribose) polymerase (PARP) degradation. Pro-caspase-9 cleavage, Bcl2 family expression changes, mitochondrial dysfunction, and intracellular ROS generation also participate in Xanthohumol-induced glioma cell death. Xanthohumol's inhibition of the IGFBP2/AKT/Bcl2 pathway via miR-204-3p targeting plays a critical role in mediating glioma cell death[4]. |
| Cell Assay | In vitro cell proliferation/viability is measured by the MTT test at different time points. 1000 cells/well are plated into 96-multiwell plates in complete medium. Following adhesion, medium is replaced with fresh medium containing the different treatments or vehicle (DMSO in medium). Xanthohumol and EGCG are used in a concentration range from 2.5 to 40 μM, up to 96 hours. 3 hours before each time point, MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is added to the wells and plates are incubated at 37°C. At the indicated time points, absorbance at 540 nm is then measured by a FLUOstar spectrophotometer. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 576.5±50.0 °C at 760 mmHg |
| Melting Point | 157-159ºC |
| Molecular Formula | C21H22O5 |
| Molecular Weight | 354.396 |
| Flash Point | 203.4±23.6 °C |
| Exact Mass | 354.146729 |
| PSA | 86.99000 |
| LogP | 5.17 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.641 |
| Storage condition | 2-8°C |
| Water Solubility | ethanol: soluble10mg/mL |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H400 |
| Precautionary Statements | P273-P280 |
| Hazard Codes | N: Dangerous for the environment; |
| Risk Phrases | R50/53 |
| Safety Phrases | 60-61 |
| RIDADR | UN 3077 9 |
| RTECS | UD5574117 |
|
Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing.
J. Nat. Prod. 76(11) , 2047-53, (2013) Type 1 diabetes mellitus is responsible for metabolic dysfunction, accompanied by chronic inflammation, oxidative stress, and endothelium dysfunction, and is often associated with impaired wound heali... |
|
|
Protective effects of arachidonic acid against palmitic acid-mediated lipotoxicity in HIT-T15 cells.
Mol. Cell Biochem. 364(1-2) , 19-28, (2012) Saturated fatty acids have been considered major contributing factors in type 2 diabetes, whereas unsaturated fatty acids have beneficial effects for preventing the development of diabetes. However, t... |
|
|
Search method for inhibitors of Staphyloxanthin production by methicillin-resistant Staphylococcus aureus.
Biol. Pharm. Bull. 35(1) , 48-53, (2012) Staphyloxanthin, a yellow pigment produced by methicillin-resistant Staphylococcus aureus (MRSA), is a virulent factor escaping from the host immune system. A new screening method for inhibitors of st... |
| (E)-1-[2,4-dihydroxy-6-methoxy-3-(3-methylbut-2-enyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one |
| MFCD00210576 |
| (2E)-1-[2,4-Dihydroxy-6-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one |
| Xanthohumol |
| 2-propen-1-one, 1-[2,4-dihydroxy-6-methoxy-3-(3-methyl-2-butenyl)phenyl]-3-(4-hydroxyphenyl)-, (2E)- |
| 2-Propen-1-one, 1-[2,4-dihydroxy-6-methoxy-3-(3-methyl-2-buten-1-yl)phenyl]-3-(4-hydroxyphenyl)-, (2E)- |
| QR D1U1VR BQ DQ FO1 C2UY1&1 |
| (2E)-1-[2,4-Dihydroxy-6-methoxy-3-(3-methyl-2-buten-1-yl)phenyl]-3-(4-hydroxyphenyl)-2-propen-1-one |

