CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XH9800000
-
CHEMICAL NAME :
-
4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-(2-phenyl acetamido)-, monosodium salt
-
CAS REGISTRY NUMBER :
-
69-57-8
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
17
-
MOLECULAR FORMULA :
-
C16-H17-N2-O4-S.Na
-
MOLECULAR WEIGHT :
-
356.40
-
WISWESSER LINE NOTATION :
-
T45 ANV ESTJ CMV1R& F1 F1 GVQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6916 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - tremor Behavioral - convulsions or effect on seizure threshold Gastrointestinal - other changes
-
REFERENCE :
-
AMPMAR Archives des Maladies Professionnelles de Medecine du Travail et de Securite Sociale. (SPPIF, B.P.22, F-41353 Vineuil, France) V.7- 1946- Volume(issue)/page/year: 39,259,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3020 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - other changes Lungs, Thorax, or Respiration - respiratory depression
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 9,31,1959
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2900 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
AACHAX Antimicrobial Agents and Chemotherapy (1961-70). (Ann Arbor, MI) 1961-70. For publisher information, see AMACCQ. Volume(issue)/page/year: -,863,1965
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ABANAE Antibiotics Annual. (New York, NY) 1953-60. For publisher information, see AMACCQ. Volume(issue)/page/year: 3,540,1955/1956
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3314 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ABANAE Antibiotics Annual. (New York, NY) 1953-60. For publisher information, see AMACCQ. Volume(issue)/page/year: 3,540,1955/1956
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4750 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NYKZAU Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. (Nippon Yakuri Gakkai, c/o Kyoto Daigaku Igakubu Yakurigaku Kyoshitsu, Konoe-cho, Yoshida, Sakyo-ku, Kyoto 606, Japan) V.40- 1944- Volume(issue)/page/year: 55,23,1959
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 9,31,1959
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2800 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 9,31,1959
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intracerebral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3800 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
NYKZAU Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. (Nippon Yakuri Gakkai, c/o Kyoto Daigaku Igakubu Yakurigaku Kyoshitsu, Konoe-cho, Yoshida, Sakyo-ku, Kyoto 606, Japan) V.40- 1944- Volume(issue)/page/year: 55,23,1959
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
150 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Nutritional and Gross Metabolic - other changes
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 9,31,1959
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
60 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
LBASAE Laboratory Animal Science. (American Assoc. for Laboratory Animal Science, 210 N. Hammes Ave., Suite 205, Joliet, IL 60435) V.21- 1971- Volume(issue)/page/year: 30,524,1980 ** TUMORIGENIC DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1840 mg/kg/46W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - lymphoma, including Hodgkin's disease Tumorigenic - tumors at site of application
-
REFERENCE :
-
BJCAAI British Journal of Cancer. (Macmillan Press Ltd., Houndmills, Basingstoke, Hants. RG21 2XS, UK) V.1- 1947- Volume(issue)/page/year: 15,85,1961 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1500 mg/kg
-
SEX/DURATION :
-
female 6-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
ANTBAL Antibiotiki. (Moscow, USSR) V.1-29, 1956-84. For publisher information, see AMBIEH. Volume(issue)/page/year: 18,815,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1500 mg/kg
-
SEX/DURATION :
-
female 1-5 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
ANTBAL Antibiotiki. (Moscow, USSR) V.1-29, 1956-84. For publisher information, see AMBIEH. Volume(issue)/page/year: 18,815,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 158,528,1964 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4380 No. of Facilities: 137 (estimated) No. of Industries: 1 No. of Occupations: 5 No. of Employees: 7315 (estimated) No. of Female Employees: 5985 (estimated)
|
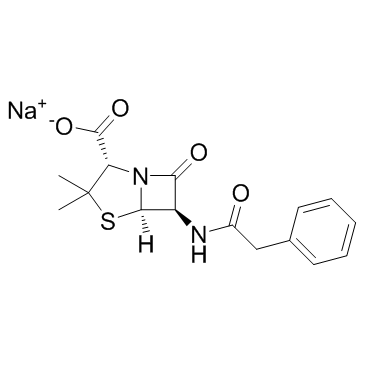


 CAS#:643-43-6
CAS#:643-43-6 CAS#:67099-38-1
CAS#:67099-38-1
