Indole-3-carbinol
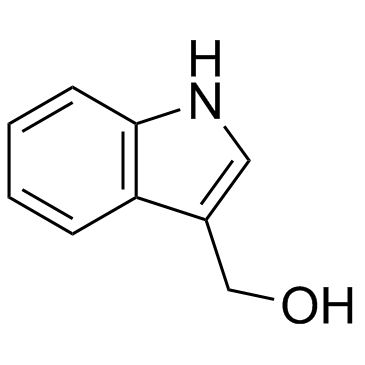
Indole-3-carbinol structure
|
Common Name | Indole-3-carbinol | ||
|---|---|---|---|---|
| CAS Number | 700-06-1 | Molecular Weight | 147.174 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 360.6±17.0 °C at 760 mmHg | |
| Molecular Formula | C9H9NO | Melting Point | 96-99 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 171.9±20.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Indole-3-carbinolIndole-3-carbinol suppresses NF-κB activity and also is an Aryl hydrocarbon receptor (AhR) agonist. |
| Name | indole-3-methanol |
|---|---|
| Synonym | More Synonyms |
| Description | Indole-3-carbinol suppresses NF-κB activity and also is an Aryl hydrocarbon receptor (AhR) agonist. |
|---|---|
| Related Catalog | |
| Target |
NF-κB AhR |
| In Vitro | It is found that Indole-3-carbinol (I3C) inhibits the proliferation of THP-1 cells in a dose- and time dependent manner with minimal toxicity over normal monocytes. The AhR target genes (CYP1A1, IL1β) are overexpressed upon Indole-3-carbinol treatment (p<0.05 to p<0.001). The antiproliferative effects of Indole-3-carbinol are in association with programing cell death. Indole-3-carbinol downregulates BCL2 and upregulates FasR in THP-1 cells (p<0.05 to p<0.001). G1 cell cycle arrest is also observed using flow cytometry. G1-acting cell cycle genes (P21, P27 and P53) are overexpressed (p<0.05 to p<0.001), while CDK2 is downregulated upon Indole-3-carbinol treatment (p<0.01 to p<0.001)[1].Indole-3-carbinol suppresses NF-κB activity and stimulates the p53 pathway in pre-B acute lymphoblastic leukemia cells[2]. |
| Cell Assay | THP-1 cells are cultured in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin and 2 mM Glutamax in a fully humidified atmosphere with 5% CO2. Cells (2-5×105 mL-1) are seeded in six well plates followed by resuspension in complete growth media. THP-1 monocyte cells are then treated with 10 ng/mL phorbol 12-myristate 13-acetate as a tumor promoter to induce stable differentiation into attaching macrophage-like cells and overexpression of AhR. The cells are then treated with varying concentrations of Indole-3-carbinol (1, 10 and 100 μM, and 1 mM). THP-1 cells and enriching normal monocytes are seeded at 5×104 cells/well in 24-well plate with different concentrations of Indole-3-carbinol and observed for 24 and 48 h followed by MTT assay. The cells are incubated in triplicates in a final volume of 200 mL of Phenol Red free RPMI 1640 for 20 h . An aliquot of 20 mL of MTT solution (5 mg/mL) is added to each well and incubated for 4 h. Formazan crystals are formed. An amount of 300 mL DMSO is then added to each well as a cell lysis solution. Percentage of cell viability is assessed by spectrophotometry at 570 nm[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 360.6±17.0 °C at 760 mmHg |
| Melting Point | 96-99 °C(lit.) |
| Molecular Formula | C9H9NO |
| Molecular Weight | 147.174 |
| Flash Point | 171.9±20.9 °C |
| Exact Mass | 147.068420 |
| PSA | 36.02000 |
| LogP | 0.96 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.705 |
| InChIKey | IVYPNXXAYMYVSP-UHFFFAOYSA-N |
| SMILES | OCc1c[nH]c2ccccc12 |
| Storage condition | 2-8°C |
| Stability | 2-80C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NL9483000 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The extended version of restriction analysis approach for the examination of the ability of low-molecular-weight compounds to modify DNA in a cell-free system.
Food Chem. Toxicol. 75 , 118-27, (2015) One of the primary requirements in toxicology is the assessment of ability of chemicals to induce DNA covalent modification. There are several well-established methods used for this purpose such as (3... |
|
|
Indole-3-carbinol inhibits tumorigenicity of hepatocellular carcinoma cells via suppression of microRNA-21 and upregulation of phosphatase and tensin homolog.
Biochim. Biophys. Acta 1853(1) , 244-53, (2015) A major obstacle to successful treatment of hepatocellular carcinoma (HCC) is its high resistance to cytotoxic chemotherapy due to overexpression of multidrug resistance genes. Activation of the AKT p... |
|
|
In vitro activity of glucosinolates and their degradation products against brassica-pathogenic bacteria and fungi.
Appl. Environ. Microbiol. 81(1) , 432-40, (2014) Glucosinolates (GSLs) are secondary metabolites found in Brassica vegetables that confer on them resistance against pests and diseases. Both GSLs and glucosinolate hydrolysis products (GHPs) have show... |
| Indole-3-carbidol |
| MFCD02683932 |
| 3-INDOLAMETHANOL |
| 3-indolemethanol |
| 1H-Indole-3-methanol (9CI) |
| Indole-3-carbinol |
| EINECS 211-836-2 |
| 3-IndoleCarbinol |
| 1H-Indol-3-ylmethanol |
| 1H-Indole-3-methanol |
| (1H-indol-3-yl)-methanol |
| (1H-indole-3-yl)-methanol |
| indole-3-methanol |
| I3C |
| 3-hydroxymethyl-1H-indole |
| 3-INDOLYLMETHANOL |
| 3-indolylcarbinol |
 CAS#:487-89-8
CAS#:487-89-8 CAS#:124-40-3
CAS#:124-40-3 CAS#:120-72-9
CAS#:120-72-9 CAS#:50-00-0
CAS#:50-00-0 CAS#:86204-54-8
CAS#:86204-54-8 CAS#:17206-03-0
CAS#:17206-03-0 CAS#:87-52-5
CAS#:87-52-5 CAS#:86204-60-6
CAS#:86204-60-6 CAS#:87-51-4
CAS#:87-51-4 CAS#:5457-31-8
CAS#:5457-31-8 CAS#:5457-28-3
CAS#:5457-28-3 CAS#:91-56-5
CAS#:91-56-5 CAS#:22546-09-4
CAS#:22546-09-4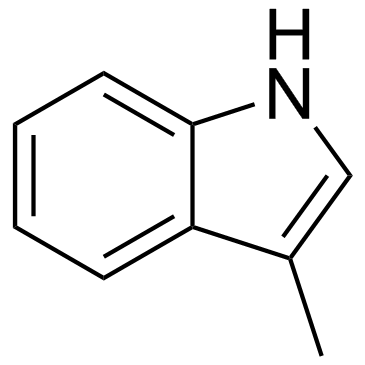 CAS#:83-34-1
CAS#:83-34-1 CAS#:4414-76-0
CAS#:4414-76-0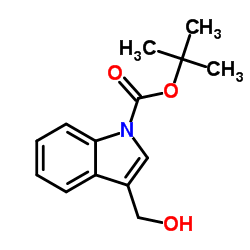 CAS#:96551-22-3
CAS#:96551-22-3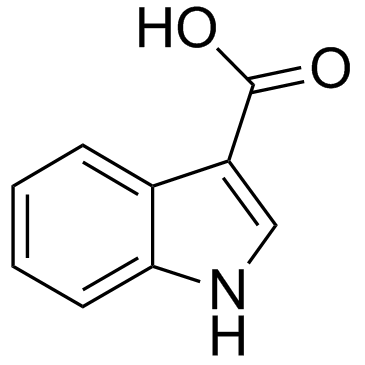 CAS#:771-50-6
CAS#:771-50-6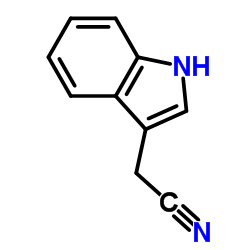 CAS#:771-51-7
CAS#:771-51-7
