| Description |
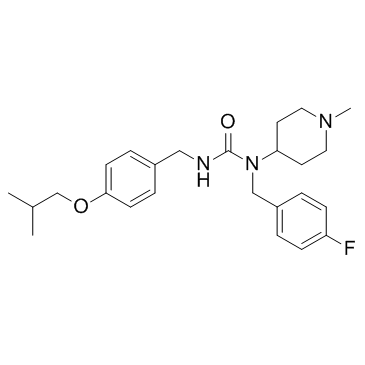
Pimavanserin is a selective inverse agonist of the 5-HT2A receptor with pIC50 and pKd of 8.73 and 9.3, respectively.
|
| Related Catalog |
|
| Target |
5-HT2A Receptor:8.7 (pIC50)
|
| In Vitro |
Pimavanserin (ACP-103) competitively antagonizes the binding of [3H]ketanserin to heterologously expressed human 5-HT2A receptors with a mean pKi of 9.3 in membranes and 9.70 in whole cells. Pimavanserin demonstrates lesser affinity (mean pKi of 8.80 in membranes and 8.00 in whole cells, as determined by radioligand binding) and potency as an inverse agonist (mean pIC50 7.1 in R-SAT) at human 5-HT2C receptors, and lacked affinity and functional activity at 5-HT2B receptors, dopamine D2 receptors, and other human monoaminergic receptors[1]. Pimavanserin (ACP-103) is highly selective for 5-HT2A receptors, lacking affinity for other receptors in a broad profile screen including 65 different molecular targets; the only other receptor for which Pimavanserin demonstrates affinity is 5-HT2C, and Pimavanserin is approximately 30-fold selective for 5-HT2A receptors over 5-HT2C receptors depending on the assay[2].
|
| In Vivo |
Pimavanserin (ACP-103) is a potent, efficacious, orally active 5-HT2A receptor inverse agonist with a behavioral pharmacological profile consistent with utility as an antipsychotic agent. Pimavanserin attenuates head-twitch behavior (3 mg/kg p.o.), and prepulse inhibition deficits (1-10 mg/kg s.c.) induced by the 5-HT2A receptor agonist (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride in rats and reduces the hyperactivity induced in mice by the N-methyl-D-aspartate receptor noncompetitive antagonist 5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine maleate; MK-801) (0.1 and 0.3 mg/kg s.c.; 3 mg/kg p.o.), consistent with a 5-HT2A receptor mechanism of action in vivo and antipsychotic-like efficacy. Pimavanserin demonstrates >42.6% oral bioavailability in rats[1].
|
| Kinase Assay |
For the membrane binding, NIH-3T3 cells are grown to 70% confluence in 15 cm2 dishes and transfected with 10 μg of receptor plasmid DNA using Polyfect transfection reagent. Two days after transfection, cells expressing the desired serotonin receptor are homogenized in 20 mM HEPES/10 mM EDTA and spun down at 11,000g at 4°C for 30 min. The supernatant is discarded, and the pellet is resuspended in 20 mM HEPES/1 mM EDTA and spun down at the same setting. The pellet is then resuspended in 20 mM HEPES/0.5 mM EDTA, and membranes are used for binding assays. Bradford analysis is used to determine total membrane protein. Kd and Bmax values are derived from 12-point concentration experiments using 1 nM [3H]ketanserin for the 5-HT2A receptor and 3 nM [3H]mesulergine for the 5-HT2B and 5-HT2C receptors. Membranes are incubated at room temperature for 3 h with various concentrations of test ligand in the presence of a fixed concentration of radioligand. The suspension is filtered as explained below for whole-cell binding, washed with ice-cold buffer, and dried, and radioactivity is determined using TopCount[1].
|
| Cell Assay |
For the whole-cell binding, 6 million human embryonic kidney 293T cells are plated in 10-cm dishes and transfected with 5 μg of plasmid DNA using Polyfect. Two days after transfection, cells are harvested with 10 mM EDTA, washed, and resuspended in binding buffer (1× DMEM with 0.1% bovine serum albumin). Then, 60,000 cells transfected with the 5-HT2A receptor or 20,000 cells transfected with the 5-HT2C-INI receptor are incubated at 37°C for 3 h in the presence of 5 nM radioligand ([3H]ketanserin for 5-HT2A receptors and [3H]mesulergine for 5-HT2C-INI receptors) and varying concentrations of ligands (total volume 100 μL in a 96-well plate). Cells are filtered onto a 96-well GF/B filter plate and washed with 300 mL of wash buffer (25 mM HEPES, 1 mM CaCl2, 5 mM MgCl2, and 0.25 M NaCl) using a Filtermate 196 harvester. The filter plates are dried under a heat lamp before addition of 50 μL of scintillation fluid to each well. Plates are counted on a TopCount. Separately, the hydrochloride salt form of Pimavanserin (10 μM) is evaluated at MDS Pharma Services for activity in a broad screen of radioligand binding assays at 65 different receptors[1].
|
| Animal Admin |
Mice[1] Non-Swiss albino mice are used for locomotor activity experiments. For determination of spontaneous activity, Pimavanserin is administered alone (s.c. 60 min before session start or p.o. 60 min before session start). For hyperactivity experiments, mice are treated with 0.3 mg/kg MK-801 (i.p.) 15 min presession (the peak dose for producing hyperactivity in an inverted-U dose-effect curve as determined in pilot experiments) in combination with vehicle or Pimavanserin. Motor activity data are collected during a 15-min session in a lit room. Mice had no prior exposure to the motor cages. Immediately before placing the mice in the locomotor chambers, effects on myorelaxation/ataxia are determined by placing each of the mouse's forepaws in contact with a horizontal wire while holding the mouse by the base of the tail. Mice are required to bring at least one hindpaw in contact with the wire within 10 s to be scored as a “pass” and failure to do so is considered ataxic. Each dose or dose combination is tested in a separate group of mice (n=8). Rats[1] For DOI head-twitch experiments in rats, vehicle or a dose of Pimavanserin is administered orally 120 min before DOI administration. DOI HCl (2.5 mg/kg i.p.) is administered immediately before observations. After injection of DOI, each rat is placed into an empty cage and observed. Latency to the first head twitch and the number of head twitches occurring over 5 min are recorded. Each rat is used only once with eight to 16 rats per dose group.
|
| References |
[1]. Vanover KE, et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inver [2]. Vanover KE, et al. A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav. 2008 Oct;90(4):540-4.
|