Denopamine
Denopamine structure
|
Common Name | Denopamine | ||
|---|---|---|---|---|
| CAS Number | 71771-90-9 | Molecular Weight | 317.38 | |
| Density | 1.177g/cm3 | Boiling Point | 518.8ºC at 760 mmHg | |
| Molecular Formula | C18H23NO4 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 267.6ºC | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of DenopamineDenopamine ((R)-(-)-Denopamine) is an orally active, selective β1-adrenergic agonist. Denopamine prolongs survival in a murine model of congestive heart failure induced by viral myocarditis: suppression of tumor necrosis factor-α production in the heart. Cardiovascular effects[1]. |
| Name | r(-)-denopamine |
|---|---|
| Synonym | More Synonyms |
| Description | Denopamine ((R)-(-)-Denopamine) is an orally active, selective β1-adrenergic agonist. Denopamine prolongs survival in a murine model of congestive heart failure induced by viral myocarditis: suppression of tumor necrosis factor-α production in the heart. Cardiovascular effects[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Denopamine (0.1-100 μM) suppresses LPS-induced TNF-α production in a concentration-dependent manner[1]. Cell Viability Assay[1] Cell Line: Murine spleen cells Concentration: 0, 0.1, 1, 10, 100 μM Incubation Time: 5 hours Result: Decreased TNF-α levels by 96.9±6.7%, 62.7±6.5%, 53.2±8.8%, and 40.3±1.5% at 0.1, 1, 10 and 100 μmol/L, respectively. |
| In Vivo | Denopamine (14 μmol/kg per day; oral administration; for 14 days) significantly improves the survival of the animals, attenuates myocardial lesions, and suppresses TNF-α production in vivo[1]. The plasma concentration of Denopamine is 13.1±1.9 nmol/L at 1 h, 4.3±0.9 nmol/L at 2 h, 1.8±0.5 nmol/L at 3 h, and <0.6 nmol/L at 5 h after its administration. A single 14 μmol/kg dose of denopamine in mice produces a peak level at 1 h[1]. Animal Model: Four-week-old inbred male DBA/2 mice[1] Dosage: 14 μmol/kg per day Administration: Oral administration; 14 days Result: Treatment significantly improved the survival of the animals (14 of 25 (56%) treated, vs 5 of 25 (20%) control mice). At day 14, the survival rate of 57.1% (16 of 28 mice) in the treated group was significantly higher than the 33.3% (10 of 30 mice) survival rate in the control group. The survival rate from day 6 to day 14 was also significantly improved in the treated group (69.6%; 16 of 23 mice) versus the control group (45.5%; 10 of 22 mice, p < 0.05). |
| References |
| Density | 1.177g/cm3 |
|---|---|
| Boiling Point | 518.8ºC at 760 mmHg |
| Molecular Formula | C18H23NO4 |
| Molecular Weight | 317.38 |
| Flash Point | 267.6ºC |
| Exact Mass | 317.16300 |
| PSA | 70.95000 |
| LogP | 2.66600 |
| Index of Refraction | 1.581 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H361 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R62 |
| Safety Phrases | S22;S45;S36/S37/S39 |
| RIDADR | NONH for all modes of transport |
| RTECS | DA4795470 |
|
Binding pockets of the beta(1)- and beta(2)-adrenergic receptors for subtype-selective agonists.
Mol. Pharmacol. 56 , 875-885, (1999) We examined the subtype-selective binding site of the beta-adrenergic receptors (betaARs). The beta(1)/beta(2)-chimeric receptors showed the importance of the second and seventh transmembrane domains ... |
|
|
Regioselective glucuronidation of denopamine: marked species differences and identification of human udp-glucuronosyltransferase isoform.
Drug Metab. Dispos. 33(3) , 403-12, (2005) Denopamine is one of the oral beta(1)-adrenoceptor-selective partial agonists. Denopamine glucuronide is the most abundant metabolite in human, rat, and dog urine when administered orally. Species dif... |
|
|
Vascular responses to beta-adrenoceptor subtype-selective agonists with and without endothelium in rat common carotid arteries.
J. Auton. Pharmacol. 21(1) , 7-13, (2001) 1. Using the cannula inserting method, vasodilator responses to beta-adrenoceptor agonists (isoprenaline, denopamine and procaterol) were investigated in isolated and perfused rat common carotid arter... |
| kalgut |
| (R)-4-{2-[2-(3,4-dimethoxy-phenyl)-ethylamino]-1-hydroxy-ethyl}-phenol |
| TA 064 |
| MFCD00867122 |
| Carguto |
| (R)-1-(4-Hydroxyphenyl)-2-(3,4-dimethoxyphenethylamino)ethanol |
| (-)-(R)-1-(p-hydroxyphenyl)-2-<(3,4-dimethoxyphenethyl)amino>ethanol |
| (-)-Denopamine |
| (R)-(-)-denopamine |
| (-)-(R)-1-(p-hydroxyphenyl)-2-<(3,4-dimehoxyphenethyl)amino>ethanol |
![(R)-1-(p-benzyloxyphenyl)-2-[2-(3,4-dimethoxyphenyl)ethylamino]ethanol Structure](https://www.chemsrc.com/caspic/347/63365-60-6.png) CAS#:63365-60-6
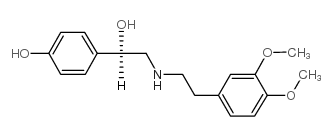
CAS#:63365-60-6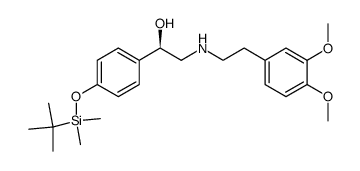 CAS#:444806-78-4
CAS#:444806-78-4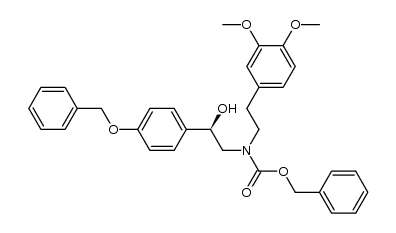 CAS#:151323-98-7
CAS#:151323-98-7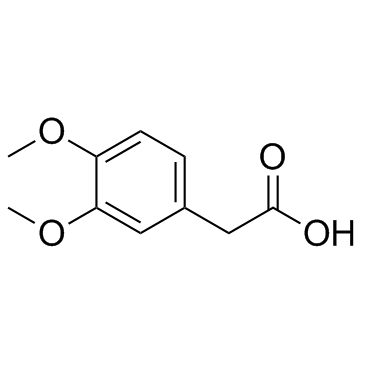 CAS#:93-40-3
CAS#:93-40-3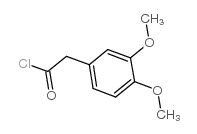 CAS#:10313-60-7
CAS#:10313-60-7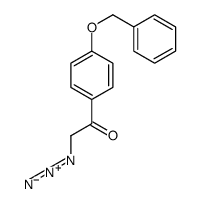 CAS#:831196-83-9
CAS#:831196-83-9![4-[(tert-Butyldimethylsilyl)oxy]benzaldehyde Structure](https://www.chemsrc.com/caspic/194/120743-99-9.png) CAS#:120743-99-9
CAS#:120743-99-9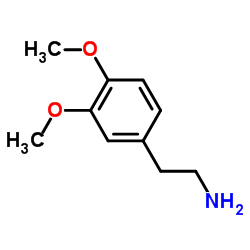 CAS#:120-20-7
CAS#:120-20-7