H-Tic-OH
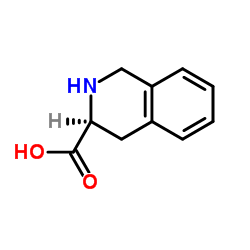
H-Tic-OH structure
|
Common Name | H-Tic-OH | ||
|---|---|---|---|---|
| CAS Number | 74163-81-8 | Molecular Weight | 177.20 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 372.0±42.0 °C at 760 mmHg | |
| Molecular Formula | C10H11NO2 | Melting Point | 300ºC | |
| MSDS | Chinese USA | Flash Point | 178.8±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of H-Tic-OHL-Porretine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | (S)-(-)-1,2,3,4-Tetrahydroisoquinoline-3-Carboxylic Acid |
|---|---|
| Synonym | More Synonyms |
| Description | L-Porretine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 一种抗酶促降解的受限苯丙氨酸类似物。 |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 372.0±42.0 °C at 760 mmHg |
| Melting Point | 300ºC |
| Molecular Formula | C10H11NO2 |
| Molecular Weight | 177.20 |
| Flash Point | 178.8±27.9 °C |
| Exact Mass | 177.078979 |
| PSA | 49.33000 |
| LogP | 0.86 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.577 |
| Storage condition | 2~8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933499090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Acid catalysis in the formation of dioxopiperazines from peptides containing tetrahydroisoquinoline-3-carboxylic acid at position 2.
Int. J. Pept. Protein Res. 45(6) , 567-73, (1995) The kinetics of the spontaneous formation of 2,5-dioxopiperazines from peptides containing the Tic (1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) residue in the 2-position of the sequence has been... |
|
|
2-Substituted (S)-2-(3,3-dimethyl-1-oxo-10,10a-dihydroimidazo[1,5-b]isoquinolin-2(1H,3H,5H)-yl)acetic acids: Conformational prediction, synthesis, anti-thrombotic and vasodilative evaluation.
Bioorg. Med. Chem. 19 , 871-82, (2011) (S)-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid (TIC) can inhibit thrombosis by inhibiting platelet aggregation. The investigation of amino acids modified TIC reveals that a stretching conformati... |
|
|
Constrained phenylalanine analogues. Preferred conformation of the 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) residue.
Int. J. Pept. Protein Res. 40 , 222, (1992) Three Tic-containing (Tic = 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) model peptides were synthesized to assess the tendency of this constrained Phe analogue to fold into a beta-bend and a hel... |
| (3S)-1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid |
| 3-Isoquinolinecarboxylic acid, 1,2,3,4-tetrahydro-, (3S)- |
| (3S)-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid |
| MFCD00800357 |
| H-Tic-OH |
| L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid |
 CAS#:50-00-0
CAS#:50-00-0 CAS#:63-91-2
CAS#:63-91-2 CAS#:77497-96-2
CAS#:77497-96-2![(10aS)-3,3-Bis(trifluoromethyl)-1-oxo-(1,2,3,4-tetrahydroisoquinolino)[2,3-c]oxazolidine Structure](https://image.chemsrc.com/caspic/073/367952-43-0.png) CAS#:367952-43-0
CAS#:367952-43-0![(3R,10bS)-3-(tert-butyl)-2-methyl-2,3,5,6-tetrahydroimidazo[5,1-a]isoquinolin-1(10bH)-one Structure](https://image.chemsrc.com/caspic/071/123053-49-6.png) CAS#:123053-49-6
CAS#:123053-49-6 CAS#:143767-56-0
CAS#:143767-56-0 CAS#:67123-97-1
CAS#:67123-97-1 CAS#:91-13-4
CAS#:91-13-4 CAS#:150582-52-8
CAS#:150582-52-8 CAS#:612-12-4
CAS#:612-12-4 CAS#:79261-58-8
CAS#:79261-58-8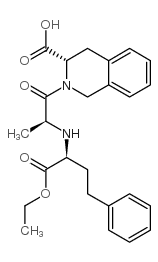 CAS#:85441-61-8
CAS#:85441-61-8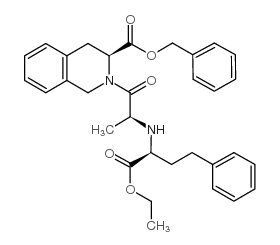 CAS#:82586-54-7
CAS#:82586-54-7 CAS#:77497-74-6
CAS#:77497-74-6 CAS#:6624-49-3
CAS#:6624-49-3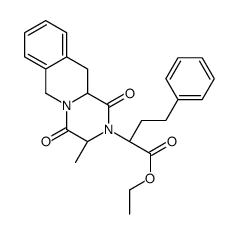 CAS#:103733-49-9
CAS#:103733-49-9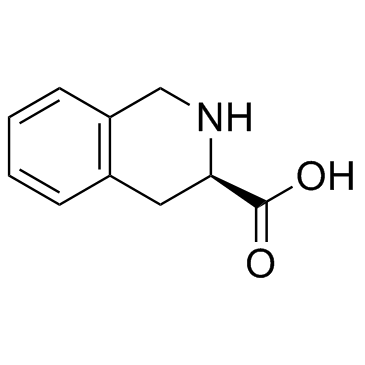 CAS#:103733-65-9
CAS#:103733-65-9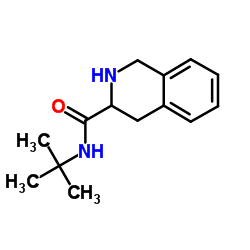 CAS#:149182-72-9
CAS#:149182-72-9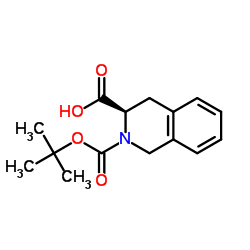 CAS#:115962-35-1
CAS#:115962-35-1
