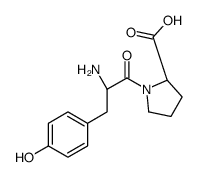
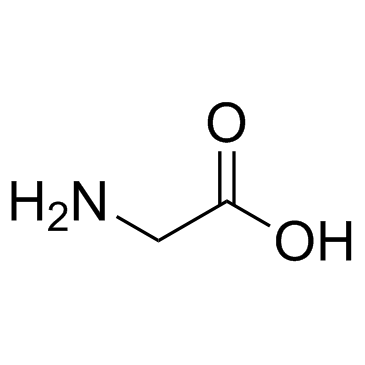
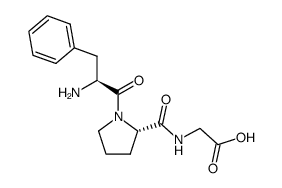
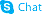Phe-Pro
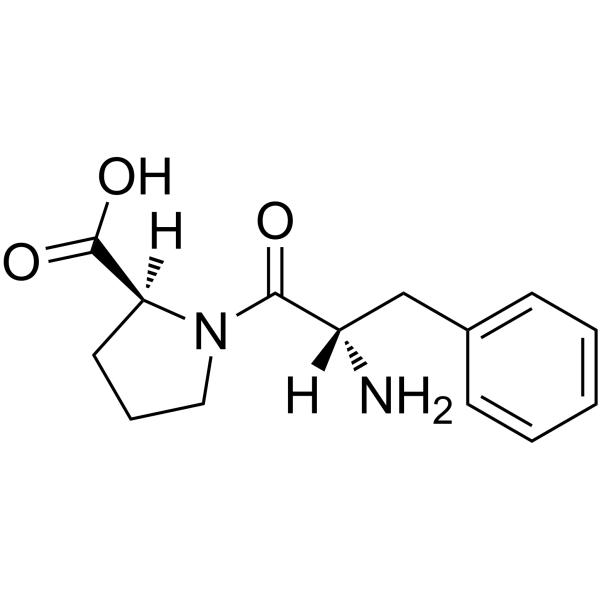
Phe-Pro structure
|
Common Name | Phe-Pro | ||
|---|---|---|---|---|
| CAS Number | 7669-65-0 | Molecular Weight | 262.30400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C14H18N2O3 | Melting Point | 127-129℃ | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Phe-Pro(S)-1-((S)-2-Amino-3-phenylpropanoyl)pyrrolidine-2-carboxylic acid is a proline derivative[1]. |
| Name | 1-(2-amino-3-phenylpropanoyl)pyrrolidine-2-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-1-((S)-2-Amino-3-phenylpropanoyl)pyrrolidine-2-carboxylic acid is a proline derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Melting Point | 127-129℃ |
|---|---|
| Molecular Formula | C14H18N2O3 |
| Molecular Weight | 262.30400 |
| Exact Mass | 262.13200 |
| PSA | 83.63000 |
| LogP | 1.27020 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3.0 |
| HS Code | 2933990090 |
|
~% 
Phe-Pro CAS#:7669-65-0 |
| Literature: Xiang, Dao Feng; Patskovsky, Yury; Xu, Chengfu; Fedorov, Alexander A.; Fedorov, Elena V.; Sisco, Abby A.; Sauder, J. Michael; Burley, Stephen K.; Almo, Steven C.; Raushel, Frank M. Biochemistry, 2010 , vol. 49, # 31 p. 6791 - 6803 |
|
~% 
Phe-Pro CAS#:7669-65-0
Detail
|
| Literature: Yan; Ho; Hou Bioscience, biotechnology, and biochemistry, 1992 , vol. 56, # 5 p. 704 - 707 |
|
~% 
Phe-Pro CAS#:7669-65-0
Detail
|
| Literature: Heiduschka; Dittrich; Heins; Neubert; Barth Pharmazie, 1989 , vol. 44, # 11 p. 778 - 780 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Determination of the cis-trans isomerization barrier of several L-peptidyl-L-proline dipeptides by dynamic capillary electrophoresis and computer simulation.
Electrophoresis 22 , 2409-2415, (2001) Dynamic capillary electrophoresis (DCE) and computer simulation of the elution profiles with the theoretical plate and the stochastic model has been applied to determine the isomerization barriers of ... |
|
|
Biological evaluation of RBx-0128, a potent and selective dipeptidyl peptidase-IV inhibitor in type 2 diabetes genetic model.
Indian J. Pharmacol. 44(6) , 759-64, (2012) Dipeptidyl peptidase IV (DPP-IV) inhibition to modulate the incretin effect is a proven strategy to treat type 2 diabetes mellitus. The present study describes the pharmacological profile of a novel D... |
|
|
Determination of the cis-trans isomerization barrier of enalaprilat by dynamic capillary electrophoresis and computer simulation.
Electrophoresis 25 , 318-323, (2004) Dynamic capillary electrophoresis (DCE) and computer simulation of the elution profiles with the stochastic model has been applied to determine the isomerization barriers of the angiotensin converting... |
| L-phenylalanyl-L-proline |
| Phe-Pro |
| Phenylalanylproline |
| H-Phe-Pro-OH |
| L-Phe-L-Pro |
| L-phenylalanine-L-Proline |