Glufosinate ammonium
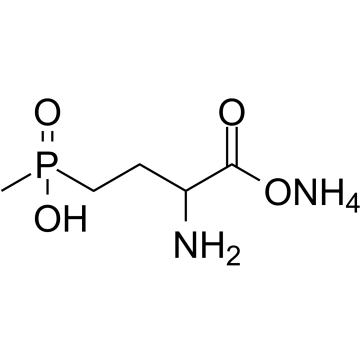
Glufosinate ammonium structure
|
Common Name | Glufosinate ammonium | ||
|---|---|---|---|---|
| CAS Number | 77182-82-2 | Molecular Weight | 215.18800 | |
| Density | N/A | Boiling Point | 519.1ºC at 760 mmHg | |
| Molecular Formula | C5H15N2O4P | Melting Point | 210°C | |
| MSDS | Chinese USA | Flash Point | 100 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of Glufosinate ammoniumGlufosinate ammonium, a phosphinic acid analogue of glutamic acid, is an herbicide which is converted by plant cells into PT (L-phosphinothricin). Glufosinate ammonium exerts neurotoxic activity[1][2]. |
| Name | glufosinate-ammonium |
|---|---|
| Synonym | More Synonyms |
| Description | Glufosinate ammonium, a phosphinic acid analogue of glutamic acid, is an herbicide which is converted by plant cells into PT (L-phosphinothricin). Glufosinate ammonium exerts neurotoxic activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Glufosinate ammonium (1-100 μM; 12DIV) disrupts cell-cell adhesion in differentiated V-SVZ neural stem cells (NSCs)[2]. Glufosinate ammonium (1-100 μM; 12DIV) significantly decreases Celsr2 gene expression at 100 μM[2]. Glufosinate ammonium impairs ependymal cell capability to synthetize cilia[2]. RT-PCR[2] Cell Line: Ependymal cells Concentration: 1, 3, 10, 100 μM Incubation Time: During 12DIV (NSCs-to-ependymal cells time point) Result: Significantly decreased Celsr2 gene expression at 100 μM. |
| In Vivo | Glufosinate ammonium (10-250 mg/kg; gavage; Daily; on days 6-15 of gestation) indicates maternal toxicity in the groups given 50 or 250 mg/kg in Wistar rats[3]. The Elimination of Glufosinate ammonium (oral intubation) in the blood takes place with a half-life of less than 4 hours in male and female rats[3]. |
| References |
| Boiling Point | 519.1ºC at 760 mmHg |
|---|---|
| Melting Point | 210°C |
| Molecular Formula | C5H15N2O4P |
| Molecular Weight | 215.18800 |
| Flash Point | 100 °C |
| Exact Mass | 215.10300 |
| PSA | 116.09000 |
| LogP | 0.24480 |
| InChIKey | ZBMRKNMTMPPMMK-UHFFFAOYSA-N |
| SMILES | CP(=O)(O)CCC(N)C(=O)O.N |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302 + H312 + H332-H360Fd-H373 |
| Precautionary Statements | P201-P260-P280-P301 + P312 + P330-P308 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 53-45 |
| RIDADR | 2588 |
| RTECS | EK7713600 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2931901917 |
|
~% 
Glufosinate ammonium CAS#:77182-82-2 |
| Literature: WO2008/138088 A2, ; Page/Page column 8-9 ; |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
|
A review of methods for the analysis of orphan and difficult pesticides: glyphosate, glufosinate, quaternary ammonium and phenoxy acid herbicides, and dithiocarbamate and phthalimide fungicides.
J. AOAC Int. 97(4) , 965-77, (2014) This article reviews the chromatography/MS methodologies for analysis of pesticide residues of orphan and difficult chemical classes in a variety of sample matrixes including water, urine, blood, and ... |
|
|
Repression of flowering under a noninductive photoperiod by the HDA9-AGL19-FT module in Arabidopsis.
New Phytol. 206(1) , 281-94, (2015) Posttranslational acetylation of histones is reversibly regulated by histone deacetylases (HDACs). Despite the evident significance of HDACs in Arabidopsis development, the biological roles and underl... |
|
|
Role of glutamate receptors and nitric oxide on the effects of glufosinate ammonium, an organophosphate pesticide, on in vivo dopamine release in rat striatum.
Toxicology 311(3) , 154-61, (2013) The purpose of the present work was to assess the possible role of glutamatergic receptors and nitric oxide (NO) production on effects of glufosinate ammonium (GLA), an organophosphate pesticide struc... |
| Glufosinate Ammonium |
| 2-Amino-4-(hydroxymethylphosphinyl)butyric acid ammonium salt |
| 2-Amino-4-(hydroxymethylphosphinyl)butanoic acid, monoammonium salt |
| DL-Phosphinothricin, monoammonium salt |
| 2-Amino-4-(hydroxymethylphosphinyl)butanoic acid monoammonium salt |
| 2-Amino-4-[hydroxy(methyl)phosphoryl]butanoic acid ammoniate (1:1) |
| Glufosinate ammonium salt |
| Ammonium (3-amino-3-carboxypropyl)methylphosphinate |
| ammonium (2RS)-2-amino-4-(methylphosphinato)butyric acid |
| Monoammonium 2-amino-4-(hydroxymethylphosphinyl)butanoate |
| Ammonium 2-amino-4-(hydroxymethylphosphinyl)butyrate |
| Basta |
| Monoammonium 4-[hydroxy(methyl)phosphinoyl]-DL-homoalaninate |
| 2-Amino-4-[hydroxy(methyl)phosphoryl]butanoic acid ammoniate |
| QVYZ2PQO&1 &&(DL) Form NH4 Salt |
| Ammonium (DL-homoalanine-4-yl)methylphosphinate |
| Glufosinate-ammonium |
| MFCD00055562 |
| Butanoic acid, 2-amino-4-(hydroxymethylphosphinyl)-, ammonium salt |
| Ammonium 2-amino-4-[hydroxy(methyl)phosphoryl]butanoate |
| glufosinate-ammonum |
| EINECS 278-636-5 |
 CAS#:73634-73-8
CAS#:73634-73-8
