(-)-Fenchone
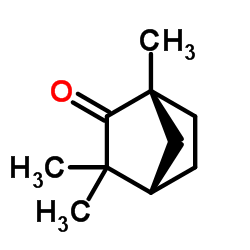
(-)-Fenchone structure
|
Common Name | (-)-Fenchone | ||
|---|---|---|---|---|
| CAS Number | 7787-20-4 | Molecular Weight | 152.233 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 193.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H16O | Melting Point | 5ºC | |
| MSDS | Chinese USA | Flash Point | 52.8±0.0 °C | |
| Symbol |

GHS02 |
Signal Word | Warning | |
Use of (-)-Fenchone(-)-Fenchone, a bicyclic monoterpene, is widely distributed in plants and found in essential oils from Thuja occidentalis. (-)-Fenchone is oxidized to 6-endo-hydroxyfenchone, 6-exo-hydroxyfenchone and 10-hydroxyfenchone derivatives by CYP2A6 and CYP2B6 in human liver microsomes with CYP2A6 playing a more important role than CYP2B6[1]. |
| Name | (1R,4S)-fenchone |
|---|---|
| Synonym | More Synonyms |
| Description | (-)-Fenchone, a bicyclic monoterpene, is widely distributed in plants and found in essential oils from Thuja occidentalis. (-)-Fenchone is oxidized to 6-endo-hydroxyfenchone, 6-exo-hydroxyfenchone and 10-hydroxyfenchone derivatives by CYP2A6 and CYP2B6 in human liver microsomes with CYP2A6 playing a more important role than CYP2B6[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 193.5±0.0 °C at 760 mmHg |
| Melting Point | 5ºC |
| Molecular Formula | C10H16O |
| Molecular Weight | 152.233 |
| Flash Point | 52.8±0.0 °C |
| Exact Mass | 152.120117 |
| PSA | 17.07000 |
| LogP | 2.13 |
| Vapour Pressure | 0.5±0.3 mmHg at 25°C |
| Index of Refraction | 1.485 |
| Storage condition | Flammables area |
| Symbol |

GHS02 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H226 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Risk Phrases | R10 |
| Safety Phrases | S16 |
| RIDADR | UN 1224 3/PG 3 |
| WGK Germany | 3 |
| RTECS | RB7875000 |
| Packaging Group | III |
| Hazard Class | 3.0 |
|
~99% 
(-)-Fenchone CAS#:7787-20-4 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 59, # 12 p. 1570 - 1573 |
|
~84% 
(-)-Fenchone CAS#:7787-20-4 |
| Literature: Journal of Chemical Research, , # 7 p. 411 - 414 |
|
~% 
(-)-Fenchone CAS#:7787-20-4 |
| Literature: Journal of Photochemistry and Photobiology A: Chemistry, , vol. 218, # 1 p. 41 - 47 |
|
~58% 
(-)-Fenchone CAS#:7787-20-4 |
| Literature: Tetrahedron Letters, , vol. 31, # 22 p. 3151 - 3154 |
|
[Chemical composition of essential oil obtained from Romanian fennel fruits].
Rev. Med. Chir. Soc. Med. Nat. Iasi. 115(2) , 590-4, (2011) For therapeutical purposes, fennel (Foeniculum vulgare Mill.), an important aromatic plant, is used for its expectorant, antispasmodic, carminative and diuretic properties. The chemical composition, e... |
|
|
Biosynthesis of monoterpenes: conversion of the acyclic precursors geranyl pyrophosphate and neryl pyrophosphate to the rearranged monoterpenes fenchol and fenchone by a soluble enzyme preparation from fennel (Foeniculum vulgare).
Arch. Biochem. Biophys. 200(2) , 524-33, (1980)
|
|
|
Kinetics and mechanisms of the tropospheric reactions of menthol, borneol, fenchol, camphor, and fenchone with hydroxyl radicals (OH) and chlorine atoms (Cl).
J. Phys. Chem. A 116(16) , 4097-107, (2012) Relative kinetic techniques have been used to measure the rate coefficients for the reactions of oxygenated terpenes (menthol, borneol, fenchol, camphor, and fenchone) and cyclohexanol with hydroxyl r... |
| (1R,4S)-1,3,3-Trimethylbicyclo[2.2.1]heptan-2-one |
| EINECS 232-107-5 |
| MFCD00151104 |
| (1R)-(-)-Fenchone |
| 2-Norbornanone, 1,3,3-trimethyl-, (1R,4S)-(+)- |
| (-)-1,3,3-Trimethyl-2-norbornanone |
| Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl-, (1R,4S)- |
| (+)-FENCHONE |
| (1R,4S)-fenchone |
![(1R,4S)-1,3,3-trimethylbicyclo[2.2.1]heptane-2-one oxime structure](https://image.chemsrc.com/caspic/286/74163-80-7.png)
![Bicyclo[2.2.1]heptane, 2-diazo-1,3,3-trimethyl-, (1R,4S) structure](https://image.chemsrc.com/caspic/070/89050-88-4.png)
![3-ethynyl-2,2,4-trimethylbicyclo[2.2.1]heptan-3-ol structure](https://image.chemsrc.com/caspic/064/18084-01-0.png)
![(1S,3R,4R)-2,2,4-trimethyl-3-pyridin-2-ylbicyclo[2.2.1]heptan-3-ol structure](https://image.chemsrc.com/caspic/020/191402-03-6.png) CAS#:191402-03-6
CAS#:191402-03-6 CAS#:137255-07-3
CAS#:137255-07-3![(1S,3R,4R)-2,2,4-trimethyl-3-phenylbicyclo[2.2.1]heptan-3-ol structure](https://image.chemsrc.com/caspic/276/161276-73-9.png) CAS#:161276-73-9
CAS#:161276-73-9
