Atomoxetine
Modify Date: 2025-08-25 17:48:32
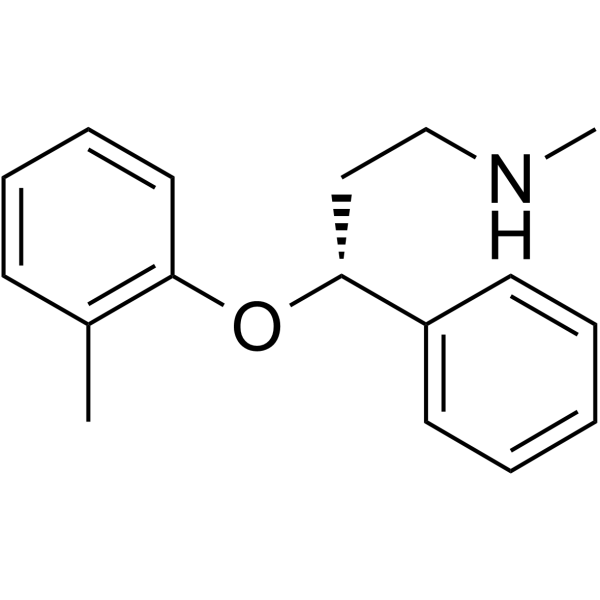
Atomoxetine structure
|
Common Name | Atomoxetine | ||
|---|---|---|---|---|
| CAS Number | 83015-26-3 | Molecular Weight | 255.355 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 389.0±37.0 °C at 760 mmHg | |
| Molecular Formula | C17H21NO | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 164.1±16.0 °C | |
Use of AtomoxetineAtomoxetine (Tomoxetine) is a selective noradrenaline reuptake inhibitor with Ki values of 5, 77 and 1451 nM for norepinephrine (NE), serotonin (5-HT) and dopamine (DA) transporters, respectively. Atomoxetine (Tomoxetine) increases of DAEX and NEEX in the PFC and enhances catecholaminergic neurotransmission. Atomoxetine (Tomoxetine) is a potent Na+ channels (VGSCs) blocker. Atomoxetine (Tomoxetine) can be used for attention-deficit hyperactivity disorder (ADHD) research[1][2][3]. |
| Name | atomoxetine |
|---|---|
| Synonym | More Synonyms |
| Description | Atomoxetine (Tomoxetine) is a selective noradrenaline reuptake inhibitor with Ki values of 5, 77 and 1451 nM for norepinephrine (NE), serotonin (5-HT) and dopamine (DA) transporters, respectively. Atomoxetine (Tomoxetine) increases of DAEX and NEEX in the PFC and enhances catecholaminergic neurotransmission. Atomoxetine (Tomoxetine) is a potent Na+ channels (VGSCs) blocker. Atomoxetine (Tomoxetine) can be used for attention-deficit hyperactivity disorder (ADHD) research[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Atomoxetine (Tomoxetine) (1-100 µM; 0.5-20 seconds; tsA201 cells) interacts with the human heart muscle sodium channel (hNav1.5) in a state and dose-dependent manner[2]. |
| In Vivo | Atomoxetine (Tomoxetine) (0.3-3 mg/kg; i.p.; 0-4 hours; male Sprague-Dawley rats) increases extracellular norepinephrine and dopamine by 3-fold and increases Fos expression in the rat prefrontal cortex[1]. Atomoxetine (Tomoxetine) (0.1-5 mg/kg; i.p. and p.o; for 14 days; spontaneously hypertensive rat) can improve behaviors associated with ADHD in rats[3]. Animal Model: Male Sprague-Dawley rats[1] Dosage: 0.3, 1 and 3 mg/kg Administration: Intraperitoneal injection; for 4 hours Result: Increased the number of cells expressing Fos-like immunoreactivity in PFC 3.7-fold and increased extracellular norepinephrine and dopamine by 3-fold. Animal Model: Spontaneously hypertensive rat (SHR)[3] Dosage: 0.1, 0.3, 1.25 and 5.0 mg/kg Administration: Intraperitoneal injection and oral administration; for 14 days Result: Had non-impact on the measurement of motor activity. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 389.0±37.0 °C at 760 mmHg |
| Molecular Formula | C17H21NO |
| Molecular Weight | 255.355 |
| Flash Point | 164.1±16.0 °C |
| Exact Mass | 255.162308 |
| PSA | 21.26000 |
| LogP | 3.28 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.552 |
| InChIKey | VHGCDTVCOLNTBX-QGZVFWFLSA-N |
| SMILES | CNCCC(Oc1ccccc1C)c1ccccc1 |
| Safety Phrases | S22-S24/25 |
|---|---|
| HS Code | 2922299090 |
| HS Code | 2922299090 |
|---|---|
| Summary | 2922299090. other amino-naphthols and other amino-phenols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| Hismanal(R) |
| [3H]-Astemizole |
| MFCD00865352 |
| benzenepropanamine, N-methyl-g-(2-methylphenoxy)-, (gR)- |
| Laridal |
| (3R)-N-Methyl-3-(2-methylphenoxy)-3-phenyl-1-propanamine |
| Histaminos |
| astemizole |
| 1-[(4-fluorophenyl)methyl]-N-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl]-1H-benzimidazole-2-amine |
| Paralergin |
| (gR)-N-Methyl-g-(2-methylphenoxy)benzenepropanamine |
| tomoxetine [INN_en] |
| 1-(4-fluorophenylmethyl)-N-{1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl}-1H-benzimidazol-2-amine |
| [14C]-Atomoxetine |
| (R)-N-methyl-3-(2-tolyloxy)-3-(phenyl)propylamine |
| (-)-Tomoxetine |
| Tomoxetine [INN] |
| Retolen |
| Alermizol |
| [14C]-Astemizole |
| (R)-Tomoxetine |
| (3R)-N-methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine |
| Atomoxetine |
| Astemison |
| Astemizolum |
| Benzenepropanamine, N-methyl-γ-(2-methylphenoxy)-, (γR)- |
| Astemizol |
| Hismanal |