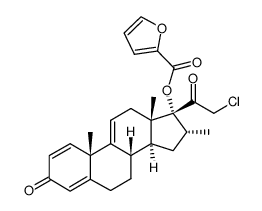
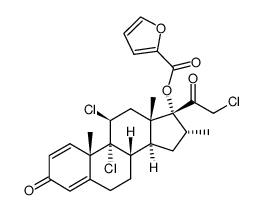
Mometasone furoate
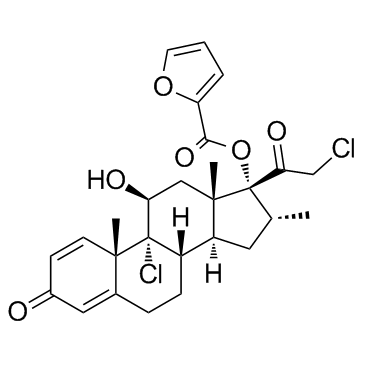
Mometasone furoate structure
|
Common Name | Mometasone furoate | ||
|---|---|---|---|---|
| CAS Number | 83919-23-7 | Molecular Weight | 521.43 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 655.5±55.0 °C at 760 mmHg | |
| Molecular Formula | C27H30Cl2O6 | Melting Point | 218-220°C | |
| MSDS | Chinese USA | Flash Point | 350.2±31.5 °C | |
Use of Mometasone furoateMometasone furoate, prodrug of the free form mometasone, is a agent with high affinity for the glucocorticoid receptor.IC50 value: Target: glucocorticosteroid receptorMometasone furoate is used in the treatment of inflammatory skin disorders (such as eczema and psoriasis), allergic rhinitis (such as hay fever), asthma [1]. MF is approved for once or bid maintenance treatment of asthma (in patients previously receiving ICS or bronchodilators). Low systemic bioavailability and high relative binding affinity for the glucocorticoid receptor are properties of MF that allow for a favourable efficacy and tolerability profile. Inhaled MF has been shown to be an effective and well-tolerated controller medication for those patients with mild, moderate or severe persistent asthma [2]. |
| Name | mometasone furoate |
|---|---|
| Synonym | More Synonyms |
| Description | Mometasone furoate, prodrug of the free form mometasone, is a agent with high affinity for the glucocorticoid receptor.IC50 value: Target: glucocorticosteroid receptorMometasone furoate is used in the treatment of inflammatory skin disorders (such as eczema and psoriasis), allergic rhinitis (such as hay fever), asthma [1]. MF is approved for once or bid maintenance treatment of asthma (in patients previously receiving ICS or bronchodilators). Low systemic bioavailability and high relative binding affinity for the glucocorticoid receptor are properties of MF that allow for a favourable efficacy and tolerability profile. Inhaled MF has been shown to be an effective and well-tolerated controller medication for those patients with mild, moderate or severe persistent asthma [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 655.5±55.0 °C at 760 mmHg |
| Melting Point | 218-220°C |
| Molecular Formula | C27H30Cl2O6 |
| Molecular Weight | 521.43 |
| Flash Point | 350.2±31.5 °C |
| PSA | 113.02000 |
| LogP | 4.27 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.604 |
| InChIKey | WOFMFGQZHJDGCX-ZULDAHANSA-N |
| SMILES | CC1CC2C3CCC4=CC(=O)C=CC4(C)C3(Cl)C(O)CC2(C)C1(OC(=O)c1ccco1)C(=O)CCl |
| Storage condition | Store at -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | WW8223000 |
| HS Code | 2937229000 |
|
~66% 
Mometasone furoate CAS#:83919-23-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 9 p. 1581 - 1588 |
|
~85% 
Mometasone furoate CAS#:83919-23-7 |
| Literature: Draper, Richard W.; Bin, Hu; McPhail, Andrew T.; Puar, Mohindar S.; Vater, Eugene J.; Weber, Lois Tetrahedron, 1999 , vol. 55, # 11 p. 3355 - 3364 |
|
~% 
Mometasone furoate CAS#:83919-23-7 |
| Literature: Tetrahedron, , vol. 55, # 11 p. 3355 - 3364 |
|
~% 
Mometasone furoate CAS#:83919-23-7 |
| Literature: Tetrahedron, , vol. 55, # 11 p. 3355 - 3364 |
|
~% 
Mometasone furoate CAS#:83919-23-7 |
| Literature: Tetrahedron, , vol. 55, # 11 p. 3355 - 3364 |
| HS Code | 2937229000 |
|---|
|
Mometasone furoate hydrogel for scalp use: in vitro and in vivo evaluation.
Pharm. Dev. Technol. 19(5) , 618-22, (2014) Dermatological inflammatory diseases often affect the scalp and the eyebrows. Common dosage forms available on the market for those situations are lotions; however, the presence of hair limits their u... |
|
|
Hypothalamic-pituitary-adrenal axis effects of mometasone furoate/formoterol fumarate vs fluticasone propionate/salmeterol administered through metered-dose inhaler.
Chest 144(6) , 1795-802, (2013) The effects of mometasone furoate and fluticasone propionate on the hypothalamic-pituitary-adrenal axis were compared when administered from combination metered-dose inhaler (MDI) products.In a random... |
|
|
Neuropathic dermatitis after flap surgery.
J. Plast. Reconstr. Aesthet. Surg. 67(7) , 1013-5, (2014)
|
| Mometasone furoate |
| (11β,16α)-9,21-Dichloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl 2-furoate |
| 9,21-Dichloro-11β,17-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17-(2-furoate) |
| (8S,9R,10S,11S,13S,14S,16R,17R)-9-Chloro-17-(chloroacetyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl 2-furoate |
| Rimelon |
| 2-furancarboxylic acid, (11b,16a)-9,21-dichloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl ester |
| Danitin |
| Mometasone furoate (JAN/USP) |
| Ecural |
| Mometasone Furoate |
| Nosorex |
| Pregna-1,4-diene-3,20-dione, 9,21-dichloro-17-((2-furanylcarbonyl)oxy)-11-hydroxy-16-methyl-, (11β,16α)- |
| (11b,16a)-9,21-Dichloro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methylpregna-1,4-diene-3,20-dione |
| (11β,16α)-9,21-Dichlor-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-ylfuran-2-carboxylat |
| (8S,9R,10S,11S,13S,14S,16R,17R)-9-Chlor-17-(chloracetyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-ylfuran-2-carboxylat |
| 9,21-Dichloro-11b,17-dihydroxy-16a-methylpregna-1,4-diene-3,20-dione17-(2-Furoate) |
| furane-2-carboxylate de (8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(chloroacétyl)-11-hydroxy-10,13,16-triméthyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodécahydro-3H-cyclopenta[a]phénanthrén-17-yle |
| Flumeta |
| [(8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloroacetyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] furan-2-carboxylate |
| MFCD00866003 |
| (11β,16α)-9,21-dichloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl furan-2-carboxylate |
| 2-Furancarboxylic acid, (11β,16α)-9,21-dichloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl ester |
| (11b,16a)-9,21-dichloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl furan-2-carboxylate |
| (8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(chloroacetyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl furan-2-carboxylate |