CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
RY4025000
-
CHEMICAL NAME :
-
Penicillanic acid, 6-phenoxyacetamido-
-
CAS REGISTRY NUMBER :
-
87-08-1
-
BEILSTEIN REFERENCE NO. :
-
0096259
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C16-H18-N2-O5-S
-
MOLECULAR WEIGHT :
-
350.42
-
WISWESSER LINE NOTATION :
-
T45 ANV ESTJ CMV1OR& F1 F1 GVQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
10 mg/kg/2D
-
TOXIC EFFECTS :
-
Liver - liver function tests impaired Skin and Appendages - dermatitis, other (after systemic exposure) Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>2220 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>2 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>1775 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>1600 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
6578 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1351 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>1775 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
822 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
1096 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - other changes
MUTATION DATA
-
TYPE OF TEST :
-
Mutation test systems - not otherwise specified
-
TEST SYSTEM :
-
Rodent - mouse Ascites tumor
-
DOSE/DURATION :
-
200 ug/L
-
REFERENCE :
-
NEOLA4 Neoplasma. (Karger-Libri, P.O. Box, CH-4009 Basel, Switzerland) V.4- 1957- Volume(issue)/page/year: 22,105,1975 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 83957 No. of Facilities: 61 (estimated) No. of Industries: 2 No. of Occupations: 6 No. of Employees: 2874 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 83957 No. of Facilities: 114 (estimated) No. of Industries: 2 No. of Occupations: 2 No. of Employees: 1964 (estimated) No. of Female Employees: 959 (estimated)
|
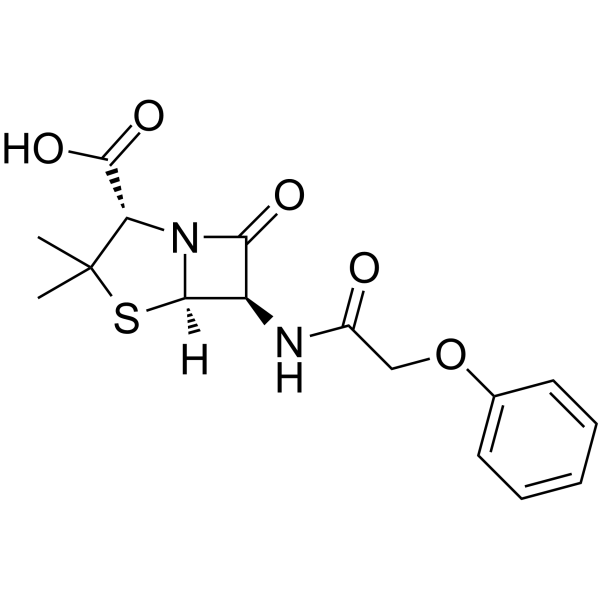
 CAS#:551-16-6
CAS#:551-16-6 CAS#:701-99-5
CAS#:701-99-5![2-bromoethyl (2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-phenoxyacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate Structure](https://image.chemsrc.com/caspic/479/65538-78-5.png) CAS#:65538-78-5
CAS#:65538-78-5 CAS#:52-67-5
CAS#:52-67-5 CAS#:1030-50-8
CAS#:1030-50-8![(4S)-2t-[(R)-tert-butoxycarbonyl-(2-phenoxy-acetylamino)-methyl]-5,5-dimethyl-thiazolidine-4r-carboxylic acid Structure](https://image.chemsrc.com/caspic/415/1057-37-0.png) CAS#:1057-37-0
CAS#:1057-37-0 CAS#:1056-69-5
CAS#:1056-69-5![2-[(1,3-dioxoisoindol-2-yl)-tert-butoxycarbonyl-methyl]-5,5-dimethyl-thiazolidine-4-carboxylic acid Structure](https://image.chemsrc.com/caspic/053/59168-65-9.png) CAS#:59168-65-9
CAS#:59168-65-9 CAS#:122-59-8
CAS#:122-59-8![methyl 3,3-dimethyl-7-oxo-6-[(2-phenoxyacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate structure](https://image.chemsrc.com/caspic/137/2315-05-1.png) CAS#:2315-05-1
CAS#:2315-05-1