CC-930
Modify Date: 2024-01-03 19:45:59
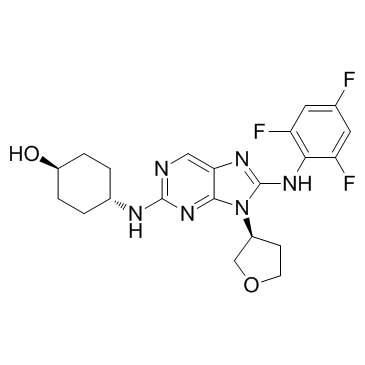
CC-930 structure
|
Common Name | CC-930 | ||
|---|---|---|---|---|
| CAS Number | 899805-25-5 | Molecular Weight | 448.441 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 626.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C21H23F3N6O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 332.6±34.3 °C | |
Use of CC-930Tanzisertib (CC-930) is a potent JNK1/2/3 inhibitor with IC50s of 61/7/6 nM, respectively. |
| Name | 4-[[9-[(3S)-oxolan-3-yl]-8-(2,4,6-trifluoroanilino)purin-2-yl]amino]cyclohexan-1-ol |
|---|---|
| Synonym | More Synonyms |
| Description | Tanzisertib (CC-930) is a potent JNK1/2/3 inhibitor with IC50s of 61/7/6 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
JNK3:6 nM (IC50) JNK2:7 nM (IC50) JNK1:61 nM (IC50) |
| In Vitro | Tanzisertib (CC-930) inhibits the formation of phospho-cJun in human PBMC stimulated by phorbol-12-myristate-13-acetate and phytohemeagglutinin (IC50=1 μM)[1]. Tanzisertib (CC-930) (1-2 μM) substantially reduces hepatocyte apoptosis and necrosis, abrogates apoptosis and necrosis in FC-loaded WT hepatocytes[2]. Tanzisertib (CC-930) blocks the JNK pathway that is activated by pro-fibrotic cytokines in systemic sclerosis[3]. |
| In Vivo | Tanzisertib (CC-930) (10 and 30 mg/kg, p.o.) inhibits the production of TNFa by 23% and 77% in the acute rat LPS-induced TNFa production PK-PD model[1]. Tanzisertib (CC-930) (150 mg/kg) prevents the development of fibrosis in different models, but can also induce the regression of pre-existing fibrosis[3]. |
| Cell Assay | Systemic sclerosis (SSc) fibroblasts are incubated with 1 µM Tanzisertib (CC-930) in 96-well plates for 20 h. Then MTT is added at a final concentration of 1 mg/mL, and the cells are further incubated at 37°C for 4 h. Mock-treated fibroblasts are used as controls, and all other results are normalised to untreated cells. |
| Animal Admin | To evaluate the regression of fibrosis on inhibition of JNK, a modified model of bleomycin-induced dermal fibrosis is used. In this model, treatment is initiated 3 weeks after the beginning of the challenge with bleomycin, when significant dermal fibrosis is already established. The outcome of six different groups with a total number of 40 mice is analysed. The first group of mice receive subcutaneous injections of NaCl for 6 weeks. The second group is injected for 3 weeks with bleomycin followed by injections of NaCl for another 3 weeks to analyse the degree of fibrosis before treatment, and to control the spontaneous regression of fibrosis. The third group of mice is killed after 6 weeks of injections with bleomycin. The fourth and the fifth group are treated with Tanzisertib (CC-930) at doses of 50 mg/kg and 150 mg/kg for the last 3 weeks of continuous challenge with bleomycin for 6 weeks. The sixth group is a positive control group consisting of mice challenged with bleomycin for 6 weeks and treated in parallel with imatinib at doses of 50 mg/kg for the last 3 weeks. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 626.4±65.0 °C at 760 mmHg |
| Molecular Formula | C21H23F3N6O2 |
| Molecular Weight | 448.441 |
| Flash Point | 332.6±34.3 °C |
| Exact Mass | 448.183472 |
| PSA | 97.12000 |
| LogP | 2.08 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.713 |
| Storage condition | -20℃ |
| CC-930 |
| trans-4-((9-((3S)-Tetrahydrofuran-3-yl)-8-((2,4,6-trifluorophenyl)amino)-9H-purin-2-yl)amino)cyclohexanol |
| Tanzisertibum |
| Tanzisertib |
| Tanzisertib (USAN) |
| trans-4-({9-[(3S)-Tetrahydro-3-furanyl]-8-[(2,4,6-trifluorophenyl)amino]-9H-purin-2-yl}amino)cyclohexanol |
| 3tti |
| Cyclohexanol, 4-[[9-[(3S)-tetrahydro-3-furanyl]-8-[(2,4,6-trifluorophenyl)amino]-9H-purin-2-yl]amino]-, trans- |