BENOXATHIAN HCL
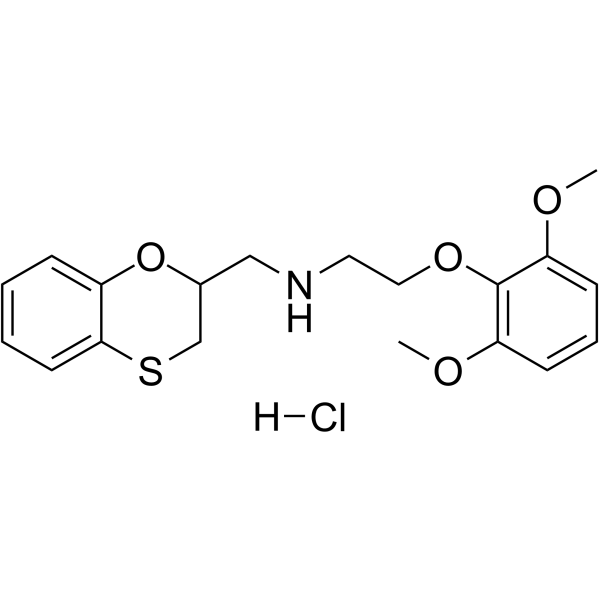
BENOXATHIAN HCL structure
|
Common Name | BENOXATHIAN HCL | ||
|---|---|---|---|---|
| CAS Number | 92642-97-2 | Molecular Weight | 397.91600 | |
| Density | N/A | Boiling Point | 500.7ºC at 760 mmHg | |
| Molecular Formula | C19H24ClNO4S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 256.6ºC | |
Use of BENOXATHIAN HCLBenoxathian hydrochloride is a potent α1 adrenoceptor antagonist, can be used for researching anorexia[1]. |
| Name | N-(2,3-dihydro-1,4-benzoxathiin-2-ylmethyl)-2-(2,6-dimethoxyphenoxy)ethanamine,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Benoxathian hydrochloride is a potent α1 adrenoceptor antagonist, can be used for researching anorexia[1]. |
|---|---|
| Related Catalog | |
| Target |
α1 Adrenoceptor[1] |
| In Vivo | Benoxathian hydrochloride (10 nM; paraventricular nucleus injection) completely reverses the anorexia induced by 2.5, 5.0 or 10.0 mg/kg Phenylpropanolamine (IP) in rats[1]. |
| References |
| Boiling Point | 500.7ºC at 760 mmHg |
|---|---|
| Molecular Formula | C19H24ClNO4S |
| Molecular Weight | 397.91600 |
| Flash Point | 256.6ºC |
| Exact Mass | 397.11100 |
| PSA | 74.25000 |
| LogP | 4.41840 |
| InChIKey | OWRADFDDJVNMGL-UHFFFAOYSA-N |
| SMILES | COc1cccc(OC)c1OCCNCC1CSc2ccccc2O1.Cl |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Liquid chromatography-quadrupole time-of-flight mass spectrometry for screening in vitro drug metabolites in humans: investigation on seven phenethylamine-based designer drugs.
J. Pharm. Biomed. Anal. 114 , 355-75, (2015) Phenethylamine-based designer drugs are prevalent within the new psychoactive substance market. Characterisation of their metabolites is important in order to identify suitable biomarkers which can be... |
|
|
α1-Adrenergic drugs modulate differentiation and cell death of human erythroleukemia cells through non adrenergic mechanism.
Exp. Cell Res. 317(16) , 2239-51, (2011) Preliminary data showed that α1-adrenergic antagonists induce apoptosis and a switch towards megakaryocytic differentiation in human erythroleukemia cells. To test the hypothesis whether survival and ... |
|
|
Activated endothelial cells limit inflammatory response, but increase chemoattractant potential and bacterial clearance by human monocytes.
Cell Biol. Int. 39 , 721-32, (2015) Inflammation is the normal immune response of vascularized tissues to damage and bacterial products, for which leukocyte transendothelial migration (TEM) is critical. The effects of cell-to-cell conta... |
| 2-[[[2-(2,6-Dimethoxyphenoxy)ethyl]-amino]-methyl]-1,4-benzoxathian hydrochloride |
| Benoxathian hydrochloride |
| Benoxathian HCl |