salicylaldehyde, oxime
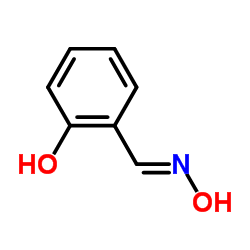
salicylaldehyde, oxime structure
|
Common Name | salicylaldehyde, oxime | ||
|---|---|---|---|---|
| CAS Number | 94-67-7 | Molecular Weight | 137.136 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 256.5±23.0 °C at 760 mmHg | |
| Molecular Formula | C7H7NO2 | Melting Point | 59-61 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 146.9±11.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of salicylaldehyde, oximeSalicylaldoxime is an organic compound, that has been used as a reagent for the gravimetric determination and separation of Cooper, Nickel, Palladium, Lead, Bismuth and Zine. The copper complex of Salicylaldoxime has anticancer activity[1][2]. |
| Name | Salicylaldoxime |
|---|---|
| Synonym | More Synonyms |
| Description | Salicylaldoxime is an organic compound, that has been used as a reagent for the gravimetric determination and separation of Cooper, Nickel, Palladium, Lead, Bismuth and Zine. The copper complex of Salicylaldoxime has anticancer activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Salicylaldoxime (300 μM) completely inhibits the relaxation activity of topoisomerase II when it forms a complex with copper; also inhibits L1210 leukaemia cell proliferation[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 256.5±23.0 °C at 760 mmHg |
| Melting Point | 59-61 °C(lit.) |
| Molecular Formula | C7H7NO2 |
| Molecular Weight | 137.136 |
| Flash Point | 146.9±11.9 °C |
| Exact Mass | 137.047684 |
| PSA | 52.82000 |
| LogP | 1.88 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.552 |
| InChIKey | ORIHZIZPTZTNCU-VMPITWQZSA-N |
| SMILES | ON=Cc1ccccc1O |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | VN5775000 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
|
Selective and potent agonists for estrogen receptor beta derived from molecular refinements of salicylaldoximes.
Eur. J. Med. Chem. 46 , 2453-62, (2011) In a continuing effort to improve the subtype selectivity and agonist potency of estrogen receptor β (ERβ) ligands, we have designed and developed a thus far unexplored structural series obtained by m... |
|
|
Structural evolutions of salicylaldoximes as selective agonists for estrogen receptor beta.
J. Med. Chem. 52 , 858-67, (2009) The bioisosteric replacement of the phenol ring, a signature functional group of most estrogen receptor (ER) ligands, with a hydrogen-bonded pseudocyclic ring, led to the development of a novel class ... |
|
|
Linking [M(III)3] triangles with "double-headed" phenolic oximes.
Dalton Trans. 41(29) , 8777-85, (2012) Strapping two salicylaldoxime units together with aliphatic α,Ω-aminomethyl links in the 3-position gives ligands which allow the assembly of the polynuclear complexes [Fe(7)O(2)(OH)(6)(H(2)L1)(3)(py)... |
| 2-hydroxybenzaldehyde oxime |
| salicylaldehyde, oxime |
| Saldox |
| Benzaldehyde, 2-hydroxy-, oxime |
| SALICYLALDEHYDE OXIME |
| Salicylaldehydoxime |
| 2-hydroxybenzaldoxime |
| SalicyladoximeGr |
| o-Hydroxybenzaldoxime |
| MFCD00002120 |
| o-hydroxybenzaldehyde oxime |
| EINECS 202-353-8 |
| 2-[(Hydroxyimino)methyl]phenol |
| Salicaldehyde oxime |
| 2-[(E)-(Hydroxyimino)methyl]phenol |
 CAS#:90-02-8
CAS#:90-02-8 CAS#:89-95-2
CAS#:89-95-2 CAS#:90-01-7
CAS#:90-01-7 CAS#:108-95-2
CAS#:108-95-2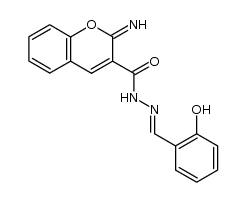 CAS#:30866-43-4
CAS#:30866-43-4 CAS#:50-00-0
CAS#:50-00-0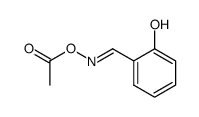 CAS#:59417-52-6
CAS#:59417-52-6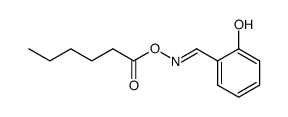 CAS#:80277-94-7
CAS#:80277-94-7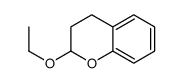 CAS#:10419-35-9
CAS#:10419-35-9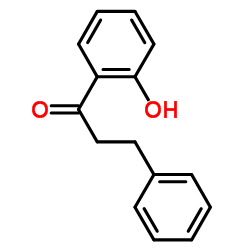 CAS#:3516-95-8
CAS#:3516-95-8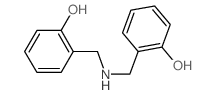 CAS#:4481-51-0
CAS#:4481-51-0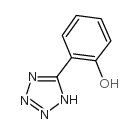 CAS#:51449-77-5
CAS#:51449-77-5 CAS#:938-73-8
CAS#:938-73-8 CAS#:487-26-3
CAS#:487-26-3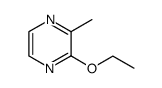 CAS#:20920-83-6
CAS#:20920-83-6 CAS#:18732-46-2
CAS#:18732-46-2 CAS#:611-20-1
CAS#:611-20-1 CAS#:271-95-4
CAS#:271-95-4
