Brompheniramine Maleate
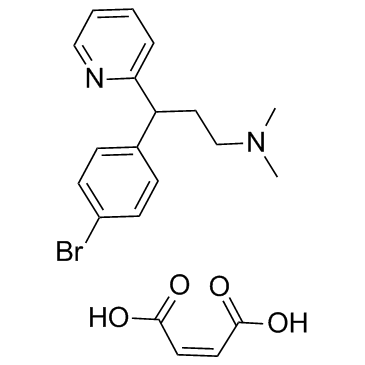
Brompheniramine Maleate structure
|
Common Name | Brompheniramine Maleate | ||
|---|---|---|---|---|
| CAS Number | 980-71-2 | Molecular Weight | 435.312 | |
| Density | N/A | Boiling Point | 403ºC at 760 mmHg | |
| Molecular Formula | C20H23BrN2O4 | Melting Point | 134-135ºC | |
| MSDS | N/A | Flash Point | 48.2 °F | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of Brompheniramine MaleateBrompheniramine maleate is a histamine H1 receptors antagonist.Target: Histamine H1 ReceptorBrompheniramine maleate, is an antihistamine drug of the propylamine (alkylamine) class. It is readily available over the counter and is indicated for the treatment of the symptoms of the common cold and allergic rhinitis. It is a first-generation antihistamine. Brompheniramine has antidepressant properties, inhibiting reuptake of the neurotransmitter serotonin. Based on this knowledge, Arvid Carlsson and his colleagues, working at the Swedish company Astra AB, were able to derive the first marketed selective serotonin reuptake inhibitor, zimelidine, from brompheniramine. Brompheniramine had a long half-life and large volume of distribution in normal adults. It also had a prolonged antihistaminic effect in the skin as evidenced by suppression of the wheal and flare response to histamine and by suppression of pruritus [1, 2]. |
| Name | brompheniramine maleate |
|---|---|
| Synonym | More Synonyms |
| Description | Brompheniramine maleate is a histamine H1 receptors antagonist.Target: Histamine H1 ReceptorBrompheniramine maleate, is an antihistamine drug of the propylamine (alkylamine) class. It is readily available over the counter and is indicated for the treatment of the symptoms of the common cold and allergic rhinitis. It is a first-generation antihistamine. Brompheniramine has antidepressant properties, inhibiting reuptake of the neurotransmitter serotonin. Based on this knowledge, Arvid Carlsson and his colleagues, working at the Swedish company Astra AB, were able to derive the first marketed selective serotonin reuptake inhibitor, zimelidine, from brompheniramine. Brompheniramine had a long half-life and large volume of distribution in normal adults. It also had a prolonged antihistaminic effect in the skin as evidenced by suppression of the wheal and flare response to histamine and by suppression of pruritus [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 403ºC at 760 mmHg |
|---|---|
| Melting Point | 134-135ºC |
| Molecular Formula | C20H23BrN2O4 |
| Molecular Weight | 435.312 |
| Exact Mass | 434.084106 |
| PSA | 90.73000 |
| LogP | 3.63950 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Hazard Codes | Xi |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | UN 3249 |
| WGK Germany | 3 |
| RTECS | US4025000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2934999090 |
| Flash Point(F) | 48.2 °F |
| Flash Point(C) | 9 °C |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Discovery of syn-/anti-cocaine-N-oxide diastereomers in unwashed postmortem hair via LC-MS-MS.
J. Anal. Toxicol. 38(6) , 360-7, (2014) The discovery of two cocaine-N-oxide (CNO) diastereomers, syn- and anti-CNO, is reported for the first time. Prior to this study, only one structural form of CNO was known to exist and has not been an... |
|
|
H1-antihistamines and oxidative burst of professional phagocytes.
Neuro Endocrinol. Lett. 30 Suppl 1 , 133-6, (2009) We analysed and compared the effect of five H1-antihistamines on stimulated oxidative burst at extra- and intracellular level of isolated and stimulated human polymorphonuclear leukocytes.Oxidative bu... |
|
|
Pheniramines and oxidative burst of blood phagocytes during ischaemia/reperfusion.
Inflamm. Res. 58 Suppl 1 , 66-7, (2009)
|
| (±)-2-[p-Bromo-a-(2-dimethylaminoethyl)benzyl]pyridine Maleate |
| D-Brompheniramine |
| dl-Bromopheniramine Maleate |
| Brompheniramine Maleate |
| (±)-Brompheniramine maleate |
| 3-(4-Bromophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine (2Z)-2-butenedioate (1:1) |
| Brompheniramine d |
| 3-(4-Bromophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine (2Z)-but-2-enedioate (1:1) |
| (+/-)-1-(4-bromophenyl)-1-(2-pyridyl)-3-dimethylaminopropane |
| 2-pyridinepropanamine, g-(4-bromophenyl)-N,N-dimethyl-, (2Z)-2-butenedioate (1:1) |
| Ilvin Maleate |
| (RS)-brompheniramine |
| MFCD00057367 |
| EINECS 213-562-9 |
| PARABROMODYLAMINE MALEATE |
| brompheniramine hydrogen maleate |
| 2-Pyridinepropanamine, γ-(4-bromophenyl)-N,N-dimethyl-, (2Z)-2-butenedioate (1:1) |
| acide (2Z)-but-2-ènedioïque - 3-(4-bromophényl)-N,N-diméthyl-3-pyridin-2-ylpropan-1-amine (1:1) |
| 3-(4-bromophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine (2Z)-but-2-enedioate |
| (2Z)-But-2-endisäure--3-(4-bromphenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amin(1:1) |
| brompheniramine (maleate) |
| N-(3-THIOXO-3H-1,2,4-DITHIAZOL-5-YL)-2-THIOPHENECARBOXAMIDE |