Lomefloxacin hydrochloride
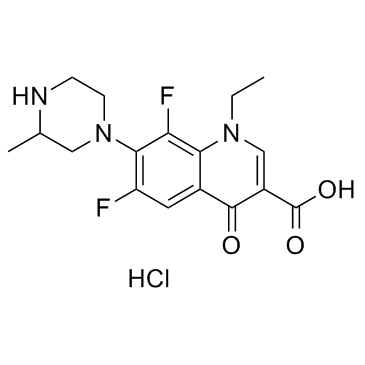
Lomefloxacin hydrochloride structure
|
Common Name | Lomefloxacin hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 98079-52-8 | Molecular Weight | 387.809 | |
| Density | N/A | Boiling Point | 542.7ºC at 760 mmHg | |
| Molecular Formula | C17H20ClF2N3O3 | Melting Point | 290-3000C | |
| MSDS | Chinese USA | Flash Point | 282ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Lomefloxacin hydrochlorideLomefloxacin HCl is a fluoroquinolone antibiotic.Target: AntibacterialLomefloxacin is a bactericidal fluoroquinolone agent with activity against a wide range of gram-negative and gram-positive organisms. The bactericidal action of lomefloxacin results from interference with the activity of the bacterial enzymes DNA gyrase and topoisomerase IV, which are needed for the transcription and replication of bacterial DNA. DNA gyrase appears to be the primary quinolone target for gram-negative bacteria. Topoisomerase IV appears to be the preferential target in gram-positive organisms. Interference with these two topoisomerases results in strand breakage of the bacterial chromosome, supercoiling, and resealing. As a result DNA replication and transcription is inhibited [1]. |
| Name | lomefloxacin hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Lomefloxacin HCl is a fluoroquinolone antibiotic.Target: AntibacterialLomefloxacin is a bactericidal fluoroquinolone agent with activity against a wide range of gram-negative and gram-positive organisms. The bactericidal action of lomefloxacin results from interference with the activity of the bacterial enzymes DNA gyrase and topoisomerase IV, which are needed for the transcription and replication of bacterial DNA. DNA gyrase appears to be the primary quinolone target for gram-negative bacteria. Topoisomerase IV appears to be the preferential target in gram-positive organisms. Interference with these two topoisomerases results in strand breakage of the bacterial chromosome, supercoiling, and resealing. As a result DNA replication and transcription is inhibited [1]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 542.7ºC at 760 mmHg |
|---|---|
| Melting Point | 290-3000C |
| Molecular Formula | C17H20ClF2N3O3 |
| Molecular Weight | 387.809 |
| Flash Point | 282ºC |
| Exact Mass | 387.116119 |
| PSA | 74.57000 |
| LogP | 2.99170 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | VB1997500 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Occurrence and distribution of antibiotics in urban soil in Beijing and Shanghai, China.
Environ. Sci. Pollut. Res. Int. 22 , 11360-71, (2015) The recycling of reclaimed wastewater for irrigation and road cleaning is an important strategy to minimize water scarcity in megacities. However, little is known regarding the potential accumulation ... |
|
|
Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater.
Water Res. 46(16) , 5235-46, (2012) This study addressed the formation and properties of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed during ozonation of secondary wastewater effluent containing these fluor... |
|
|
Real-time visualization of photochemically induced fluorescence of 8-halogenated quinolones: lomefloxacin, clinafloxacin and Bay3118 in live human HaCaT keratinocytes.
Photochem. Photobiol. 86(4) , 792-7, (2010) Halogenoquinolones are potent and widely used antimicrobials blocking microbial DNA synthesis. However, they induce adverse photoresponses through the absorption of UV light, including phototoxicity a... |
| 1-ethyl-6,8-difluoro-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid,hydrochloride |
| MFCD00214312 |
| Lomefloxacin hydrochloride |
| Lomefloxacin (hydrochloride) |

